Cancer Res Treat.
2008 Jun;40(2):81-86.
Gemcitabine and Vinorelbine Combination Chemotherapy in Anthracycline- and Taxane-pretreated Advanced Breast Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. moisa@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Abstract
-
PURPOSE: Anthracycline and taxanes are effective agents in advanced breast cancer and prolong survival times. Some patients achieve prolongation of life with capecitabine, gemcitabine, or vinorelbine, even after failure of both anthracycline and taxanes. We analyzed the efficacy and toxicity of gemcitabine and vinorelbine combination chemotherapy in anthracycline- and taxane-pretreated advanced breast cancer.
MATERIALS AND METHODS
The medical records of anthracycline- and taxane-pretreated metastatic breast cancer patients who received gemcitabine and vinorelbine combination chemotherapy at the Seoul National University Hospital were reviewed. Gemcitabine (1,000 mg/m2) and vinorelbine (25 mg/m2) were administered intravenously on days 1 and 8 every 3 weeks.
RESULTS
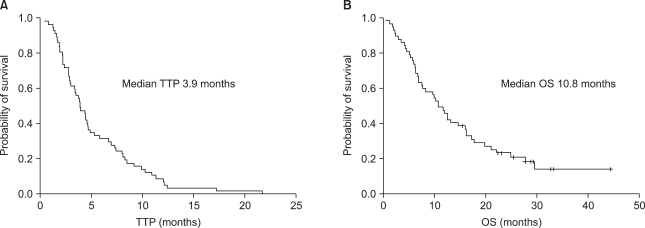
Between 2000 and 2006, 57 patients were eligible (median age, 45 years), and the median number of previous chemotherapy regimens was 3 (range, 1~5). The overall response rate was 30% (95% CI, 18.1~41.9), and the disease control rate was 46% (PR, 30%; SD, 16%). The median duration of follow-up was 33.4 months, the median time-to-progression (TTP) was 3.9 months, and the median overall survival was 10.8 months. None of thepatients with patients with anthracycline and taxane primary resistance showed a response and the median TTP for these patients was significantly shorter than that of other patients (1.9 vs. 4.4 months; p=0.018). Although the efficacy was unsatisfactory in patients with both anthracycline and taxane primary resistance, gemcitabine and vinorelbine combination chemotherapy showed comparable efficacy in anthracycline- and/or taxane-sensitive patients and the patients with secondary resistance, even after failure of second-line therapy. Grade 3/4 hematologic toxicities included neutropenia (18.1%) and febrile neutropenia (0.3%), and non-hematologic toxicities were tolerable.
CONCLUSION
Gemcitabine and vinorelbine combination chemotherapy in anthracycline- and taxane-pretreated advanced breast cancer was effective and tolerable.
Keyword
MeSH Terms
Figure
Reference
-
1. Ahn SH. Korean Breast Cancer Society. Clinical characteristics of breast cancer patients in Korea in 2000. Arch Surg. 2004; 139:27–30. PMID: 14718270.
Article2. Winer EP, Morrow M, Osborne CK, Harris JR. Devita VT, Hellman S, Rosenberg SA, editors. Malignant tumors of the breast. Cancer: principles and practice of oncology. 2004. 6th ed. Philadelphia, PA: Lippincott, Williams and Wilkins;p. 1651–1717.3. Kataja VV, Colleoni M, Bergh J. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of locally recurrent or metastatic breast cancer (MBC). Ann Oncol. 2005; 16:i10–i12. PMID: 15888735.
Article4. Plunkett W, Huang P, Searcy CE, Gandhi V. Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol. 1996; 23:3–15. PMID: 8893876.5. Carmichael J, Possinger K, Phillip P, Beykirch M, Kerr H, Walling J, et al. Advanced breast cancer: a phase II trial with gemcitabine. J Clin Oncol. 1995; 13:2731–2736. PMID: 7595731.
Article6. Potier P. The synthesis of navelbine prototype of a new series of vinblastine derivatives. Semin Oncol. 1989; 16:2–4. PMID: 2540531.7. Weber BL, Vogel C, Jones S, Harvey H, Hutchins L, Bigley J, et al. Intravenous vinorelbine as first-line and second-line therapy in advanced breast cancer. J Clin Oncol. 1995; 13:2722–2736. PMID: 7595730.
Article8. Herbst RS, Lynch C, Vasconcelles M, Teicher BA, Strauss G, Elias A, et al. Gemcitabine and vinorelbine in patients with advanced lung cancer: Preclinical studies and report of a phase I trial. Cancer Chemother Pharmacol. 2001; 48:151–159. PMID: 11561781.
Article9. Donadio M, Ardine M, Berruti A, Ritorto G, Fea E, Mistrangelo M, et al. Gemcitabine and vinorelbine as second-line treatment in patients with metastatic breast cancer: A phase II study. Cancer Chemother Pharmacol. 2003; 52:147–152. PMID: 12764672.
Article10. Mariani G, Tagliabue P, Zucchinelli P, Brambilla C, Demicheli R, Villa E, et al. Phase I/II study of gemcitabine in association with vinorelbine for metastatic breast cancer. Breast Cancer Res Treat. 2001; 70:163–169. PMID: 11804180.
Article11. Nicolaides C, Dimopoulos MA, Samantas E, Bafaloukos D, Kalofonos C, Fountzilas G, et al. Gemcitabine and vinorelbine as second-line treatment in patients with metastatic breast cancer progressing after first-line taxane-based chemotherapy: A phase II study conducted by the hellenic cooperative oncology group. Ann Oncol. 2000; 11:873–875. PMID: 10997817.
Article12. Rossi E, Perrone F, Labonia V, Landi G, Nuzzo F, Amabile G, et al. Is gemcitabine plus vinorelbine active in second-line chemotherapy of metastatic breast cancer? A single-center phase 2 study. Oncology. 2003; 64:479–480. PMID: 12759551.
Article13. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216. PMID: 10655437.14. Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE version 3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology. 2003; 13:176–181. PMID: 12903007.15. Grindey GB, Hertel LW, Plunkett W. Cytotoxicity and antitumor activity of 2', 2'-difluorodeoxycytidine (Gemcitabine). Cancer Invest. 1990; 8:313. PMID: 2400957.16. Binet S, Fellous A, Lataste H, Krikorian A, Couzinier JP, Meininger V. In situ analysis of the action of navelbine on various types of microtubules using immunofluorescence. Semin Oncol. 1989; 16:5–8. PMID: 2652320.17. Ravdin PM, Burris HA 3rd, Cook G, Eisenberg P, Kane M, Bierman WA, et al. Phase II trial of docetaxel in advanced anthracycline-resistant or anthracenedione-resistant breast cancer. J Clin Oncol. 1995; 13:2879–2885. PMID: 8523050.
Article18. Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995; 13:2886–2894. PMID: 8523051.
Article19. Verschraegen CF, Sittisomwong T, Kudelka AP, Guedes EP, Steger M, Nelson-Taylor T, et al. Docetaxel for patients with paclitaxel-resistant mullerian carcinoma. J Clin Oncol. 2000; 18:2733–2789. PMID: 10894873.20. Pivot X, Asmar L, Buzdar AU, Valero V, Hortobagyi G. A unified definition of clinical anthracycline resistance breast cancer. Brit J Cancer. 2000; 82:529–534. PMID: 10682660.
Article21. Burris H III, Yardley D, Jones S, Houston G, Broome C, Thompson D, et al. Phase II trial of trastuzumab followed by weekly paclitaxel/carboplatin as first-line treatment for patients with metastatic breast cancer. J Clin Oncol. 2004; 22:1621–1629. PMID: 15117984.
Article22. Tedesco KL, Thor AD, Johnson DH, Shyr Y, Blum KA, Goldstein LJ, et al. Docetaxel combined with trastuzumab is an active regimen in HER-2 3+ overexpressing and fluorescent in situ hybridization-positive metastatic breast cancer: a multi-institutional phase II trial. J Clin Oncol. 2004; 22:1071–1077. PMID: 15020608.
Article23. O'Shaughnessy JA, Vukelja S, Marsland T, Kimmel G, Ratnam S, Pippen JE. Phase II study of trastuzumab plus gemcitabine in chemotherapy-pretreated patients with metastatic breast cancer. Clin Breast Cancer. 2004; 5:142–147. PMID: 15245619.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gemcitabine Single or Combination Chemotherapy in Post Anthracycline and Taxane Salvage Treatment of Metastatic Breast Cancer: Retrospective Analysis of 124 Patients
- Phase II Study of Gemcitabine plus Cisplatin in Patients with Anthracycline- and Taxane- Pretreated Metastatic Breast Cancer
- Phase II Study of Gemcitabine and Vinorelbine as Second-Line Chemotherapy in Non-Small Cell Lung Cancer
- Gemcitabine Plus Vinorelbine as Second-line Chemotherapy of the Patients of Previously Treated Non-small Cell lung Cancer: Phase II Trial
- Treatment with Cisplatin and Etoposide Chemotherapy in Patient with Metastatic Breast Cancer