J Korean Acad Conserv Dent.
2005 Nov;30(6):470-476. 10.5395/JKACD.2005.30.6.470.
Regulation of pulpal microcirculation by calcitonin gene-related peptide
- Affiliations
-
- 1Department of Conservative Dentistry and Laboratory of Pulp Biology, School of Dentistry, Kyungpook National University, Korea. skykim@knu.ac.kr
- KMID: 2175753
- DOI: http://doi.org/10.5395/JKACD.2005.30.6.470
Abstract
- The purpose of this study was to investigate the function of calcitonin gene-related peptide (CGRP) in regulatory mechanism of pulpal microcirculation with the aim of elucidating neurogenic inflammation.
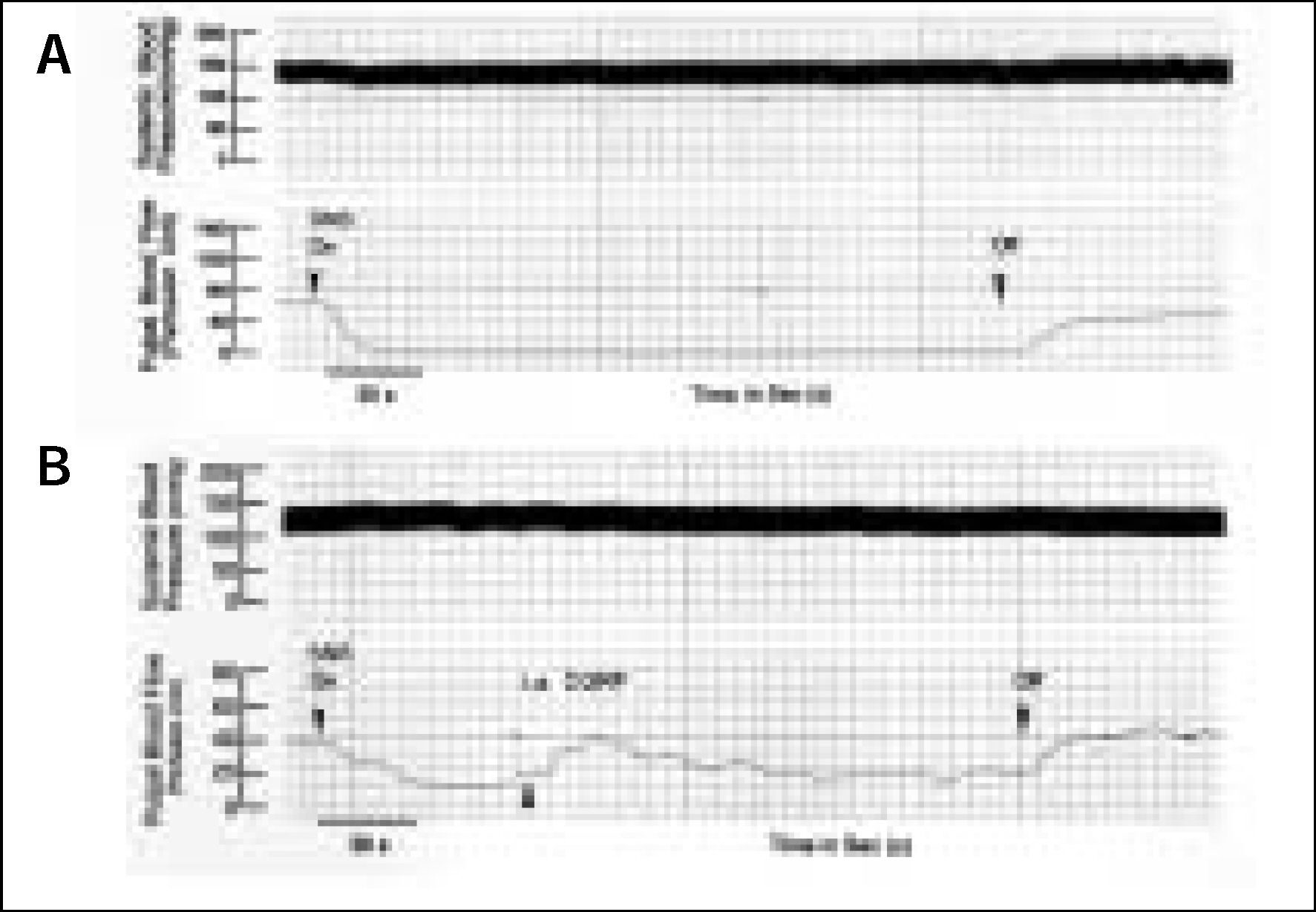
Experiments were performed on twelve cats under general anesthesia. CGRP was administered through the femoral vein to see the systemic influence and through the external carotid artery to see the local effect. Sympathetic nerve to the dental pulp was stimulated electrically and pulpal blood flow (PBF) was measured with a laser Doppler flowmeter on the canine teeth to the drug administration. The paired variables of control and experimental data were compared by paired t-test and differences with p < 0.05 were considered statistically significant.
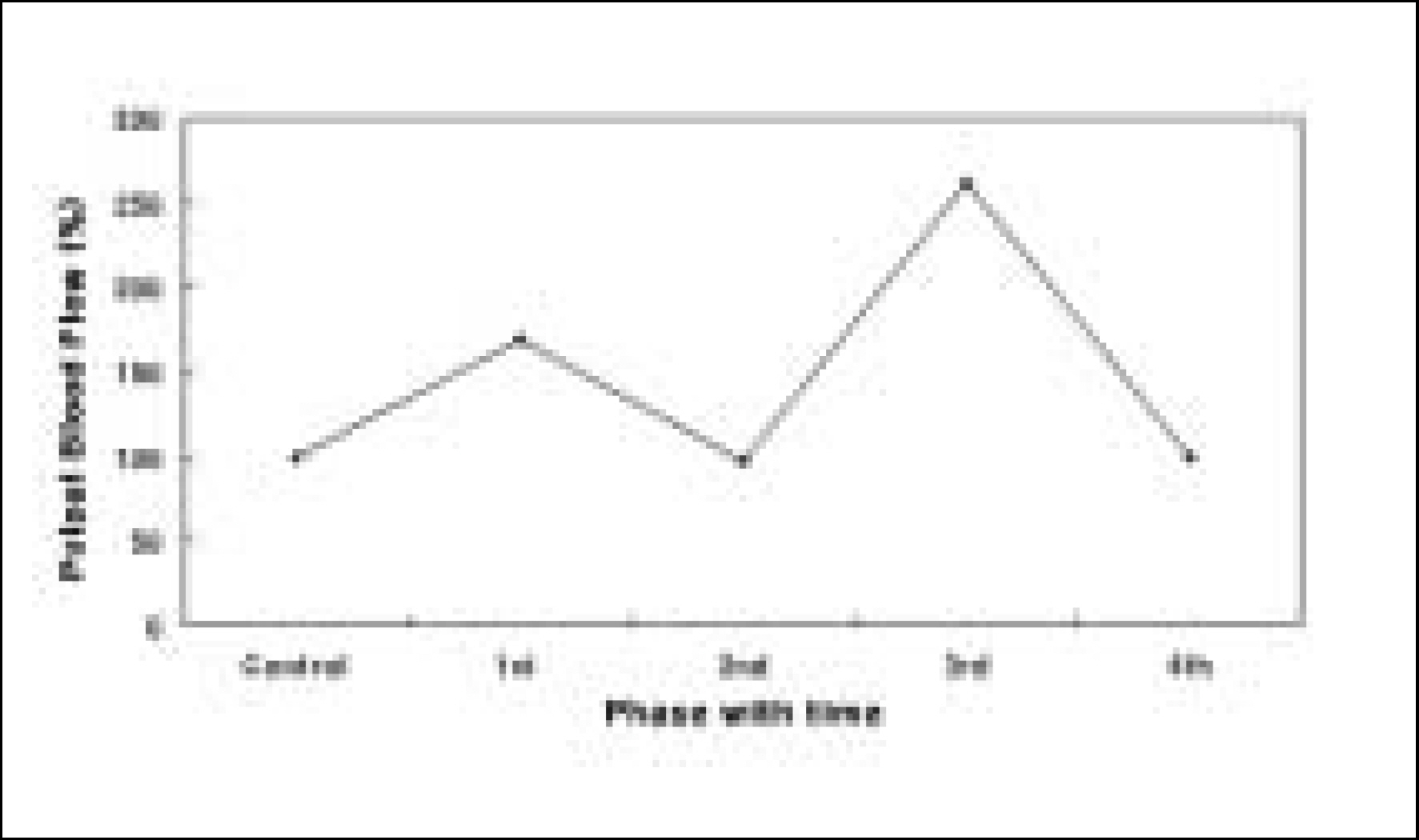
Systemic administration of CGRP (0.3 microg/kg) exerted decreases in systemic blood pressure and caused changes in PBF with an initial increase followed by decrease and a more marked second increase and decrease.
Close intra-arterial (i.a.) injection of CGRP (0.03 microg/kg) resulted in slight PBF increase. The effect of CGRP resulted in no significant increase in PBF in the presence of CGRP8-37.
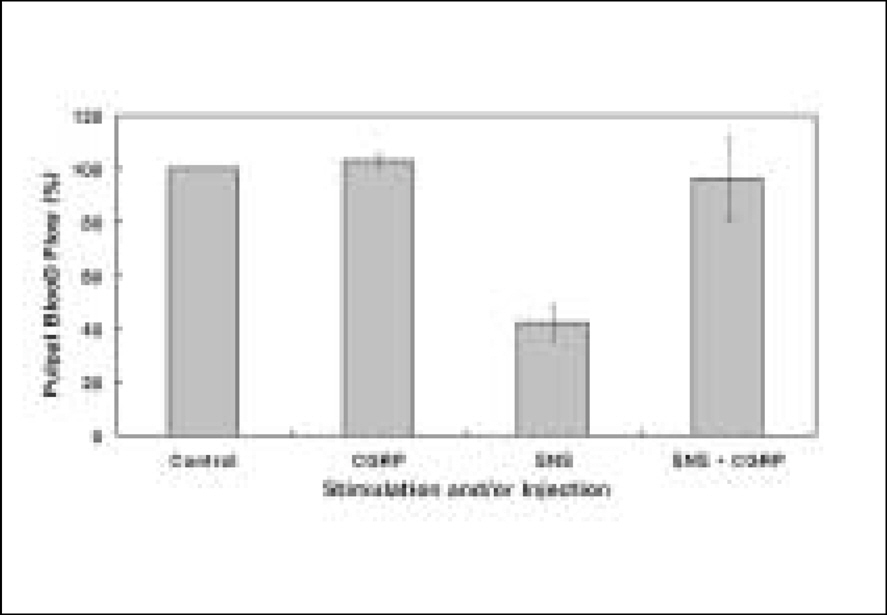
The electrical stimulation of the sympathetic nerve alone resulted in PBF decreases. The i.a. administration of CGRP following the electrical stimulation of the sympathetic nerve compensated the decreased PBF. Therefore, CGRP effectively blocked the sympathetic nerve stimulation-induced PBF decrease.
Results
of the present study have provided evidences that even though the local vasodilatory function of CGRP are weak, CGRP is effectively involved in blocking the vasoconstriction caused by sympathetic nerve stimulation in the feline dental pulp.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Goodman EC, Iversen LL. Calcitonin gene-related peptide: novel neuropeptide. Life Sci. 16(38):2169–2178. 1986.
Article2. Cao Z, Zhao M, Hu S. The change of calcitonin gene-related peptide positive fibers in inflammatory pulps. Zhonghua Kou Qiang Yi Xue Za Zhi. 32:164–166. 1997.3. Jacobsen EB, Fristad I, Heyeraas KJ. Nerve fibers immunoreactive to calcitonin gene-related peptide, substance P, neuropeptide Y, and dopamine beta-hydroxylase in innervated and denervated oral tissues in ferrets. Acta Odontol Scand. 56:220–228. 1998.4. Fristad I, Vandevska-Radunovic V, Fjeld K, Wimalawansa SJ, Hals Kvinnsland I NK. 1, NK2, NK3 and CGRP1 receptors identified in rat oral soft tissues, and in bone and dental hard tissue cells. Cell Tissue Res. 311:383–391. 2003.5. Caviedes-Bucheli J, Camargo-Beltran C, Gomez-la-Rotta AM, Moreno SC, Abello GC, Gonzalez-Escobar JM. Expression of calcitonin gene-related peptide (CGRP) in irreversible acute pulpitis. J Endod. 30:201–204. 2004.6. Kim SK. Role of sympathetic nerve on the control of microcirculation in the feline dental pulp. J Kor Acad Cons Dent. 21:375–384. 1996.7. Byers MR, Taylor PE, Khayat BG, Kimberly CL. Effect of injury and inflammation on pulpal and periapical nerves. J Endod. 16:78–84. 1990.8. Narhi M. Interaction between the autonomic and sensory nerves in the dental pulp. Proc Finn Dent Soc. 85:389–393. 1989.9. Brown MJ, Morice AH. Clinical pharmacology of vasodilator peptides. J Cardiovasc Pharmacol. 10((Suppl) 12):S82–87. 1987.
Article10. Haass M, Skofitsch G. Cardiovascular effects of calcitonin gene-related peptide in the pitched rat: comparison with substance. P. Life Sci. 2(37):2085–2090. 1985.11. Gennari C, Fischer JA. Cardiovascular action of calcitonin gene-related peptide in humans. Calcif Tissue Int. 37:581–584. 1985.
Article12. Marshall I, Al-Kazwini SJ, Roberts PM, Shepperson NB, Adams M, Craig RK. Cardiovascular effects of human and rat CGRP compared in the rat and other species. Eur J Pharmacol. 16(123):207–216. 1986.
Article13. Kim S, Fan F-c, Chen RYZ, Simchon S, Schuessler GB, Chien S. Effects of changes in systemic hemodynamic parameters on pulpal hemodynamics. J Endod. 6:394–399. 1980.14. Olgart LM. Involvement of sensory nerves in hemodynamic reactions. Proc Finn Dent Soc. 88(Suppl 1):403–410. 1992.15. Lundberg JM. Peptidergic control of the autonomic regulation system in the orofacial region. Proc Finn Dent Soc. 85:239–250. 1989.16. Maltos KL, Menezes GB, Caliari MV, Rocha OA, Santos JM, Alves DL, Duarte ID, Francischi JN. Vascular and cellular responses to pro-inflammatory stimuli in rat dental pulp. Arch Oral Biol. 49:443–450. 2004.
Article17. Kim S, Liu M, Simchon S, Do¨rscher-Kim JE. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc. 88(Suppl 1):387–392. 1992.18. Kim S, Do¨rscher-Kim J. Hemodynamic regulation of the dental pulp in a low compliance environment. J Endod. 15:404–408. 1989.
Article19. Kim SK, Ang L, Hsu YY, Do¨rscher-Kim J, Kim S. Antagonistic effect of D-myo-inositol-1, 2, 6-trisphos-phate (PP56) on the neuropeptide Y-induced vasoconstriction in the feline dental pulp. Arch Oral Biol. 41:791–798. 1996.20. Hargreaves KM, Jackson DL, Bowles WR. Adrenergic regulation of capsaicin-sensitive neurons in dental pulp. J Endod. 29:397–399. 2003.
Article21. Kerezoudis NP, Olgart L, Funato A, Edwall L. Inhibitory influence of sympathetic nerves on afferent nerve-induced extravasation in the rat incisor pulp upon direct electrical stimulation of the tooth. Arch Oral Biol. 38:483–490. 1993.
Article22. Kerezoudis NP, Funato A, Edwall L, Olgart L. Activation of sympathetic nerves exerts an inhibitory influence on afferent nerve-induced vasodilation unrelated to vasoconstriction in rat dental pulp. Acta Physiol Scand. 147:27–35. 1993.
Article23. Bowles WR, Flores CM, Jackson DL, Hargreaves KM. Beta 2-Adrenoceptor regulation of CGRP release from capsaicin-sensitive neurons. J Dent Res. 82:308–311. 2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Antiandrogen on Calcitonin Gene-related Peptide mRNA Expression ofthe Rat Cremaster Nucleus
- A immunohistochemical study of localization of calcitonin gene related peptide in the rats cochlear nucleus and superior olivary complex
- The Effect of Calcitonin Gene-Related Peptide on Hair Growth in Vitro
- Distribution of Calcitonin Gene-Related Peptide Immunoreactive Nerve Fibers in Rat Cochlea and Brainstem Auditory Nuclei
- Effect of Antiandrogen on Calcitonin Gene-related Peptide Receptor of the Rat Gubernaculum