Investig Magn Reson Imaging.
2015 Jun;19(2):99-106. 10.13104/imri.2015.19.2.99.
Factors Influencing the Background Parenchymal Enhancement in Follow-Up Breast MRI after Adjuvant Endocrine Therapy
- Affiliations
-
- 1Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. jhyouk@yuhs.ac
- KMID: 2175589
- DOI: http://doi.org/10.13104/imri.2015.19.2.99
Abstract
- PURPOSE
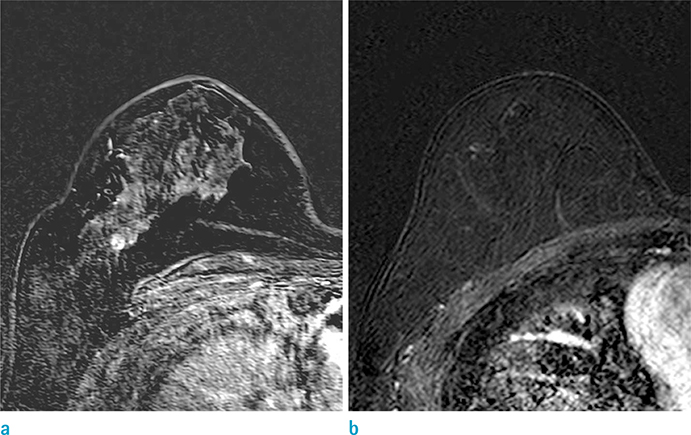
To investigate factors influencing the evaluation of background parenchymal enhancement (BPE) at follow-up breast magnetic resonance imaging (MRI) after adjuvant endocrine therapy.
MATERIALS AND METHODS
One hundred twelve women with breast cancer and MRI of the contralateral unaffected breast before and after endocrine therapy were identified. Two readers in consensus performed blinded side-by-side comparison of BPE (minimal, mild, moderate, and marked) before and after therapy with categorical scales. Age, body mass index, menopausal status, treatment regimen (selective estrogen receptor modulator or aromatase inhibitor), chemotherapy, follow-up duration, BPE at baseline MRI, MRI field strength before and after therapy, and recurrence were analyzed for their influences on decreased BPE.
RESULTS
Younger age, premenopausal status, treatment with selective estrogen receptor modulator, MRI field strength, and moderate or marked baseline BPE were significantly associated with decreased BPE. In multivariate analysis, MRI field strength and baseline BPE showed a significant association.
CONCLUSION
MRI field strength and baseline BPE before and after therapy were associated with decreased BPE at post-therapy, follow-up MRI.
MeSH Terms
-
Antineoplastic Agents
Aromatase
Body Mass Index
Breast Neoplasms
Breast*
Consensus
Drug Therapy
Estrogen Receptor Modulators
Female
Follow-Up Studies*
Humans
Magnetic Resonance Imaging*
Multivariate Analysis
Recurrence
Selective Estrogen Receptor Modulators
Weights and Measures
Antineoplastic Agents
Aromatase
Estrogen Receptor Modulators
Selective Estrogen Receptor Modulators
Figure
Reference
-
1. Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009; 9:631–643.2. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011; 378:771–784.3. Cuzick J. Breast density predicts endocrine treatment outcome in the adjuvant setting. Breast Cancer Res. 2012; 14:109.4. Kim J, Han W, Moon HG, et al. Breast density change as a predictive surrogate for response to adjuvant endocrine therapy in hormone receptor positive breast cancer. Breast Cancer Res. 2012; 14:R102.5. Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol. 2010; 195:510–516.6. Mousa NA, Eiada R, Crystal P, Nayot D, Casper RF. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause. 2012; 19:420–425.7. King V, Goldfarb SB, Brooks JD, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology. 2012; 264:670–678.8. Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrastenhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997; 203:137–144.9. Muller-Schimpfle M, Ohmenhauser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997; 203:145–149.10. Chen JH, Yu H, Lin M, Mehta RS, Su MY. Background parenchymal enhancement in the contralateral normal breast of patients undergoing neoadjuvant chemotherapy measured by DCE-MRI. Magn Reson Imaging. 2013; 31:1465–1471.11. Pfleiderer SO, Sachse S, Sauner D, et al. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res. 2004; 6:R232–R238.12. Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging--initial observations. Radiology. 2005; 235:36–41.13. Eng-Wong J, Orzano-Birgani J, Chow CK, et al. Effect of raloxifene on mammographic density and breast magnetic resonance imaging in premenopausal women at increased risk for breast cancer. Cancer Epidemiol Biomarkers Prev. 2008; 17:1696–1701.14. Oksa S, Parkkola R, Luukkaala T, Maenpaa J. Breast magnetic resonance imaging findings in women treated with toremifene for premenstrual mastalgia. Acta Radiol. 2009; 50:984–989.15. King V, Kaplan J, Pike MC, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J. 2012; 18:527–534.16. Schrading S, Schild H, Kuhr M, Kuhl C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology. 2014; 271:45–55.17. Morris E, Comstock CE, Lee CH, et al. ACR BI-RADS magnetic resonance imaging. In ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology;2013.18. Morris EA. Diagnostic breast MR imaging: current status and future directions. Radiol Clin North Am. 2007; 45:863–880. vii.19. Uematsu T, Kasami M, Watanabe J. Background enhancement of mammary glandular tissue on breast dynamic MRI: imaging features and effect on assessment of breast cancer extent. Breast Cancer. 2012; 19:259–265.20. Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med. 2010; 115:434–441.21. Harris JR, Lippman ME, Morrow M, Osborne CK. Disease of the breast. Philadelphia, PA: Lippincott Williams & Wilkins;2010.22. Raza S. Implementing a breast MR imaging program: all things considered. Magn Reson Imaging Clin N Am. 2010; 18:187–198. vii.23. Rahbar H, Partridge SC, DeMartini WB, Thursten B, Lehman CD. Clinical and technical considerations for high quality breast MRI at 3 Tesla. J Magn Reson Imaging. 2013; 37:778–790.24. Chatterji M, Mercado CL, Moy L. Optimizing 1.5-Tesla and 3-Tesla dynamic contrast-enhanced magnetic resonance imaging of the breasts. Magn Reson Imaging Clin N Am. 2010; 18:207–224. viii.25. Kuhl CK, Jost P, Morakkabati N, Zivanovic O, Schild HH, Gieseke J. Contrast-enhanced MR imaging of the breast at 3.0 and 1.5 T in the same patients: initial experience. Radiology. 2006; 239:666–676.26. Pinker K, Grabner G, Bogner W, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Invest Radiol. 2009; 44:553–558.27. Rakow-Penner R, Daniel B, Yu H, Sawyer-Glover A, Glover GH. Relaxation times of breast tissue at 1.5T and 3T measured using IDEAL. J Magn Reson Imaging. 2006; 23:87–91.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Background Parenchymal Enhancement on Breast MRI in Breast Cancer Patients : Impact on Biopsy Rate and Cancer Yield
- Effect of the Menstrual Cycle on Background Parenchymal Enhancement Observed on Breast MRIs in Korean Women
- Ultrafast Dynamic Contrast-Enhanced Breast MRI:Lesion Conspicuity and Size Assessment according toBackground Parenchymal Enhancement
- Systemic adjuvant therapy in breast cancer
- Effect of Adjuvant Hormonal Therapy on the Development of Pulmonary Fibrosis after Postoperative Radiotherapy for Breast Cancer