Diabetes Metab J.
2012 Aug;36(4):275-279. 10.4093/dmj.2012.36.4.275.
Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction via Beta Cell ABCA1
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. bscha@yuhs.ac
- 2The Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2174422
- DOI: http://doi.org/10.4093/dmj.2012.36.4.275
Abstract
- BACKGROUND
The aim of this study was to investigate the effects of balsamic vinegar on beta-cell dysfunction.
METHODS
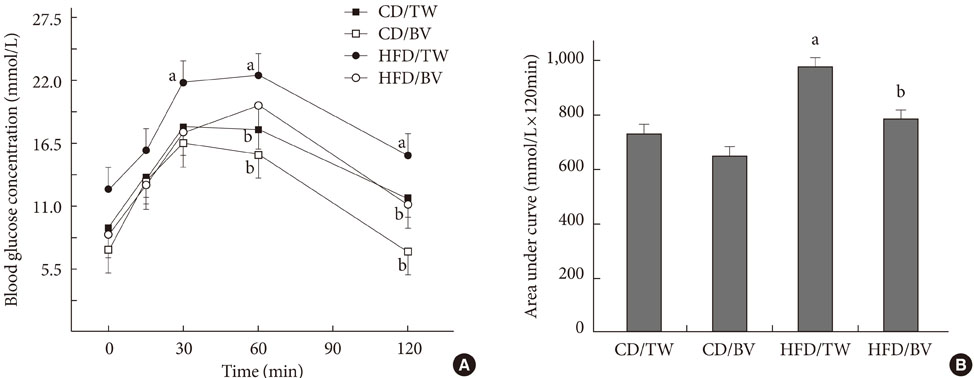
In this study, 28-week-old Otsuka Long-Evans Tokushima Fatty (OLETF) rats were fed a normal chow diet or a high-fat diet (HFD) and were provided with tap water or dilute balsamic vinegar for 4 weeks. Oral glucose tolerance tests and histopathological analyses were performed thereafter.
RESULTS
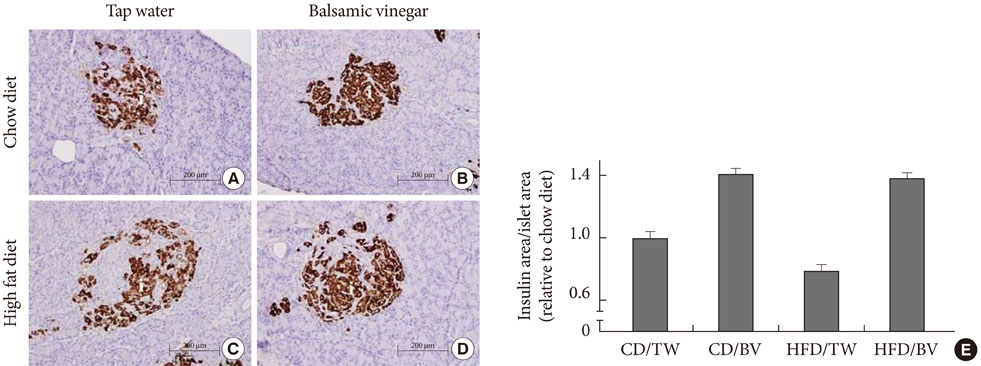
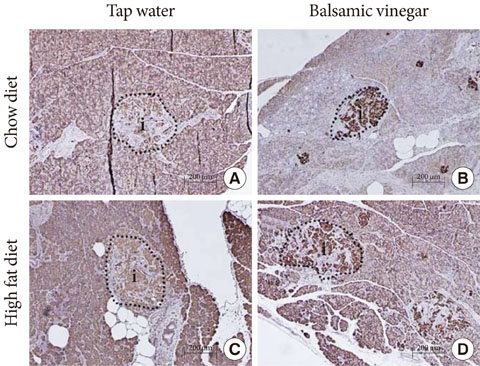
In rats fed both the both chow diet and the HFD, the rats given balsamic vinegar showed increased insulin staining in islets compared with tap water administered rats. Balsamic vinegar administration also increased beta-cell ATP-binding cassette transporter subfamily A member 1 (ABCA1) expression in islets and decreased cholesterol levels.
CONCLUSION
These findings provide the first evidence for an anti-diabetic effect of balsamic vinegar through improvement of beta-cell function via increasing beta-cell ABCA1 expression.
Keyword
MeSH Terms
Figure
Reference
-
1. Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007. 13:340–347.2. Kruit JK, Kremer PH, Dai L, Tang R, Ruddle P, de Haan W, Brunham LR, Verchere CB, Hayden MR. Cholesterol efflux via ATP-binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol-induced impairment of beta cell function in mice. Diabetologia. 2010. 53:1110–1119.3. Cnop M, Hannaert JC, Grupping AY, Pipeleers DG. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology. 2002. 143:3449–3453.4. Chakravarthy MV, Semenkovich CF. The ABCs of beta-cell dysfunction in type 2 diabetes. Nat Med. 2007. 13:241–242.5. Kruit JK, Wijesekara N, Fox JE, Dai XQ, Brunham LR, Searle GJ, Morgan GP, Costin AJ, Tang R, Bhattacharjee A, Johnson JD, Light PE, Marsh BJ, Macdonald PE, Verchere CB, Hayden MR. Islet cholesterol accumulation due to loss of ABCA1 leads to impaired exocytosis of insulin granules. Diabetes. 2011. 60:3186–3196.6. Johnston CS, Kim CM, Buller AJ. Vinegar improves insulin sensitivity to a high-carbohydrate meal in subjects with insulin resistance or type 2 diabetes. Diabetes Care. 2004. 27:281–282.7. Leeman M, Ostman E, Bjorck I. Vinegar dressing and cold storage of potatoes lowers postprandial glycaemic and insulinaemic responses in healthy subjects. Eur J Clin Nutr. 2005. 59:1266–1271.8. Kitamura T, Kido Y, Nef S, Merenmies J, Parada LF, Accili D. Preserved pancreatic beta-cell development and function in mice lacking the insulin receptor-related receptor. Mol Cell Biol. 2001. 21:5624–5630.9. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981. 29:577–580.10. Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000. 39:843–849.11. Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. Mechanisms of beta-cell death in type 2 diabetes. Diabetes. 2005. 54:Suppl 2. S108–S113.12. Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007. 56:2328–2338.13. Peyot ML, Pepin E, Lamontagne J, Latour MG, Zarrouki B, Lussier R, Pineda M, Jetton TL, Madiraju SR, Joly E, Prentki M. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010. 59:2178–2187.14. Ishikawa M, Iwasaki Y, Yatoh S, Kato T, Kumadaki S, Inoue N, Yamamoto T, Matsuzaka T, Nakagawa Y, Yahagi N, Kobayashi K, Takahashi A, Yamada N, Shimano H. Cholesterol accumulation and diabetes in pancreatic beta-cell-specific SREBP-2 transgenic mice: a new model for lipotoxicity. J Lipid Res. 2008. 49:2524–2534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction via Beta Cell ABCA1 (Diabetes Metab J 2012;36:275-9)
- Letter: Balsamic Vinegar Improves High Fat-Induced Beta Cell Dysfunction via Beta Cell ABCA1 (Diabetes Metab J 2012;36:275-9)
- The Relationship between beta-cell Function and Nutrient Intakes in Korean Adult: Using 4th Korea National Health and Nutrition Examination Survey 2009
- Beta-Cell Function and Nutrient Intake
- Therapeutic Approaches for Preserving or Restoring Pancreatic beta-Cell Function and Mass