J Gynecol Oncol.
2011 Dec;22(4):244-252. 10.3802/jgo.2011.22.4.244.
Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass
- Affiliations
-
- 1Institute of Clinical Sciences, Department of Obstetrics and Gynecology, Sahlgrenska Cancer Centre, University of Gothenburg, Gothenburg, Sweden. karin.sundfeldt@gu.se
- KMID: 2173626
- DOI: http://doi.org/10.3802/jgo.2011.22.4.244
Abstract
OBJECTIVE
Women presenting with a large or complex ovarian cyst are referred to extensive surgical staging to ensure the correct diagnosis and treatment of a possible epithelial ovarian cancer. We hypothesized that measurement of the biomarkers HE4 and CA-125 preoperatively would improve the assignment of these patients to the correct level of care.
METHODS
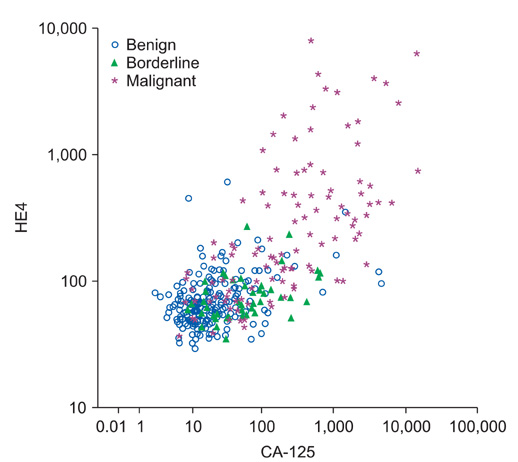
Patients diagnosed with a cystic ovarian mass and scheduled for an operation at our center of excellence for ovarian cancer surgery from 2001 to 2010 were prospectively included (n=394) and plasma was collected consecutively. Cut-off for HE4 was calculated at 75% specificity (85 pM and 71.8 pM for post and premenopausal women). For CA-125, 35 U/mL cut-off was used. The study population included women with malignant (n=114), borderline (n=45), and benign (n=215) ovarian tumors.
RESULTS
Receiver operator characteristic (ROC) area under the curve (AUC) in the benign versus malignant cohorts was 86.8% for CA-125 and 84.4% for HE4. Negative predictive value was 91.7% when at least one of the biomarkers was positive, with only early stage epithelial ovarian cancer showing false negative results. Sensitivity at set specificity (75%) was 87% for risk of ovarian malignancy algorithm (ROMA) in the postmenopausal cohort (cut-off point, 26.0%) and 81% in the premenopausal cohort (cut-off point, 17.3%). ROC AUC in the benign versus stage I epithelial ovarian cancer was only 72% for HE4 and 76% for CA-125.
CONCLUSION
In our study, population HE4 did not outperform CA-125. Based on our data a prospective trial with patients already diagnosed with an ovarian cyst may be conducted.
MeSH Terms
Figure
Reference
-
1. Granberg S, Wikland M, Jansson I. Macroscopic characterization of ovarian tumors and the relation to the histological diagnosis: criteria to be used for ultrasound evaluation. Gynecol Oncol. 1989. 35:139–144.2. van Nagell JR Jr, DePriest PD, Ueland FR, DeSimone CP, Cooper AL, McDonald JM, et al. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007. 109:1887–1896.3. Suh-Burgmann E. Long-term outcomes following conservative surgery for borderline tumor of the ovary: a large population-based study. Gynecol Oncol. 2006. 103:841–847.4. Bast RC Jr, Badgwell D, Lu Z, Marquez R, Rosen D, Liu J, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer. 2005. 15:Suppl 3. 274–281.5. Woolas RP, Xu FJ, Jacobs IJ, Yu YH, Daly L, Berchuck A, et al. Elevation of multiple serum markers in patients with stage I ovarian cancer. J Natl Cancer Inst. 1993. 85:1748–1751.6. Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast RC Jr, et al. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010. 117:440–445.7. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008. 108:402–408.8. Hough CD, Sherman-Baust CA, Pizer ES, Montz FJ, Im DD, Rosenshein NB, et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 2000. 60:6281–6287.9. Schummer M, Ng WV, Bumgarner RE, Nelson PS, Schummer B, Bednarski DW, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999. 238:375–385.10. Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003. 63:3695–3700.11. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009. 112:40–46.12. Montagnana M, Lippi G, Ruzzenente O, Bresciani V, Danese E, Scevarolli S, et al. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal. 2009. 23:331–335.13. Li J, Dowdy S, Tipton T, Podratz K, Lu WG, Xie X, et al. HE4 as a biomarker for ovarian and endometrial cancer management. Expert Rev Mol Diagn. 2009. 9:555–566.14. Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary: FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006. 95:Suppl 1. S161–S192.15. Shah CA, Lowe KA, Paley P, Wallace E, Anderson GL, McIntosh MW, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125. Cancer Epidemiol Biomarkers Prev. 2009. 18:1365–1372.16. Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008. 110:374–382.17. Fujirebio Diagnostics I. HE4 EIA package insert [Internet]. cited 2011 May 8. Goteborg: Fujirebio Diagnostics;Available from: http://www.fdi.com/documents/products/inserts/eia/HE4%20EIA%20404-10,%202008-09,%20r1.pdf.18. du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer. 2009. 115:1234–1244.19. Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990. 97:922–929.20. Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010. 203:228.e1–228.e6.21. McDonald JM, Doran S, DeSimone CP, Ueland FR, DePriest PD, Ware RA, et al. Predicting risk of malignancy in adnexal masses. Obstet Gynecol. 2010. 115:687–694.22. Scholler N, Crawford M, Sato A, Drescher CW, O'Briant KC, Kiviat N, et al. Bead-based ELISA for validation of ovarian cancer early detection markers. Clin Cancer Res. 2006. 12(7 Pt 1):2117–2124.23. Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, et al. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010. 116:378–383.24. Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011. 104:863–870.25. Abdel-Azeez HA, Labib HA, Sharaf SM, Refai AN. HE4 and mesothelin: novel biomarkers of ovarian carcinoma in patients with pelvic masses. Asian Pac J Cancer Prev. 2010. 11:111–116.26. Palmer C, Duan X, Hawley S, Scholler N, Thorpe JD, Sahota RA, et al. Systematic evaluation of candidate blood markers for detecting ovarian cancer. PLoS One. 2008. 3:e2633.27. Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009. 100:1315–1319.28. Lowe KA, Shah C, Wallace E, Anderson G, Paley P, McIntosh M, et al. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008. 17:2480–2487.29. Menon U, Jacobs IJ. Ovarian cancer screening in the general population. Curr Opin Obstet Gynecol. 2001. 13:61–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical efficacy of serum human epididymis protein 4 as a diagnostic biomarker of ovarian cancer: A pilot study
- Comparison of HE4, CA125, and Risk of Ovarian Malignancy Algorithm in the Prediction of Ovarian Cancer in Korean Women
- The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4
- Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- HE4, CA-125, and cystic ovarian mass