J Gynecol Oncol.
2009 Sep;20(3):169-175. 10.3802/jgo.2009.20.3.169.
Correlation between preoperative serum levels of five biomarkers and relationships between these biomarkers and cancer stage in epithelial overian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kangwon National University Medical School, Chuncheon, Korea. obypf@kangwon.ac.kr
- KMID: 2173450
- DOI: http://doi.org/10.3802/jgo.2009.20.3.169
Abstract
OBJECTIVE
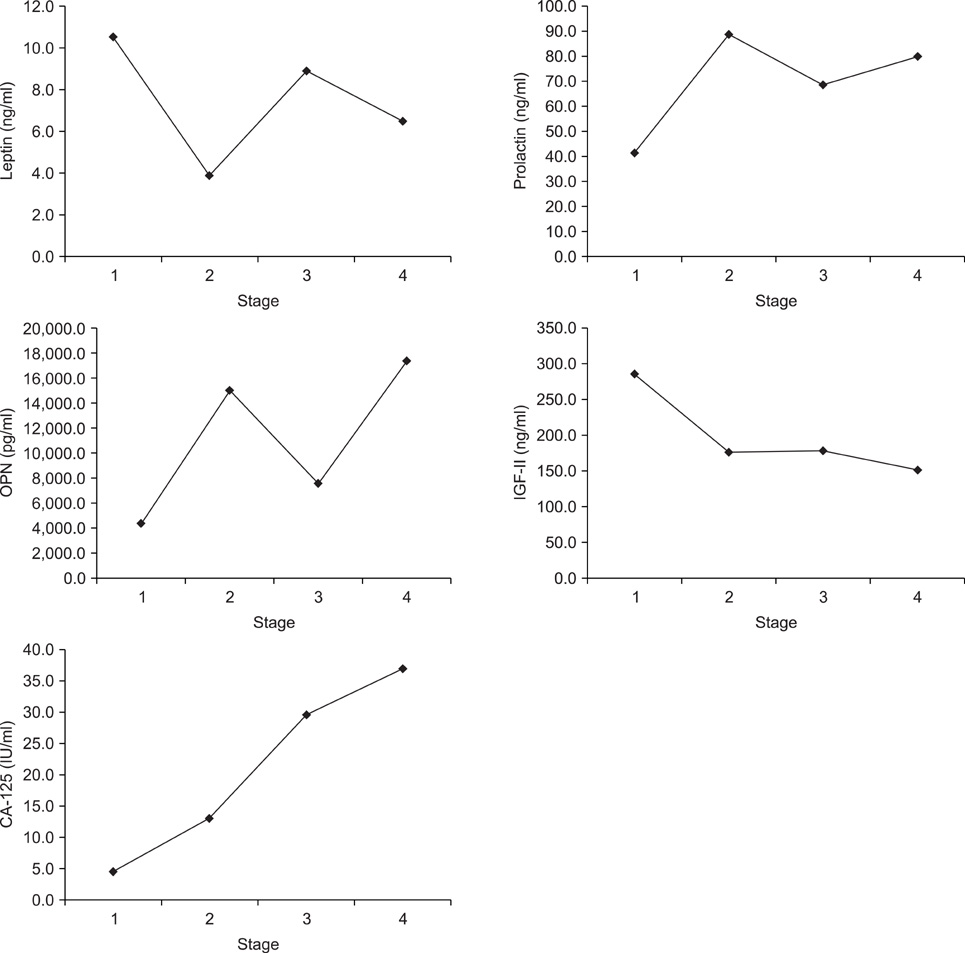
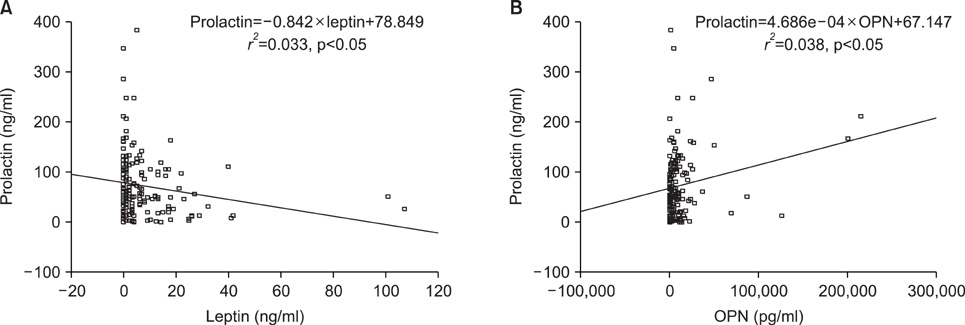
To examine the correlation among the preoperative serum levels of five biomarkers presumed to be useful for early detection of epithelial ovarian cancer and evaluate the relationships between serum levels of these five biomarkers and epithelial ovarian cancer stage. METHODS: We analyzed 56 newly diagnosed epithelial ovarian cancer patients. Preoperative serum levels of leptin, prolactin, osteopontin (OPN), insulin-like growth factor-II, and CA-125 were determined by ELISA. We also examined the correlation between the serum levels of the biomarkers and ovarian cancer stage. Significant differences in the mean serum levels of two proteins, leptin and CA-125, were observed between stage subsets. RESULTS: There was a significant negative correlation between prolactin and leptin and a significant positive correlation between prolactin and OPN. Of the five biomarkers, only the mean serum CA-125 level showed a significant positive correlation with cancer stage (Spearman rho=0.24, p<0.01). OPN showed a marginally significant positive correlation with stage (Spearman rho=0.14, p=0.07). CONCLUSION: We demonstrated the relationship between five biomarkers in epithelial ovarian cancer. These tumor markers may be useful in screening for ovarian cancer, in characterizing disease states, and in developing therapeutic interventions targeting these marker proteins. Large-scale studies that include potential confounding factors and modifiers are necessary to more accurately define the value of these novel biomarkers in ovarian cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.2. Berchuck A, Elbendary A, Havrilesky L, Rodriguez GC, Bast RC Jr. Pathogenesis of ovarian cancers. J Soc Gynecol Investig. 1994. 1:181–190.3. Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002. 107:99–118.4. Rapkiewicz AV, Espina V, Petricoin EF 3rd, Liotta LA. Biomarkers of ovarian tumours. Eur J Cancer. 2004. 40:2604–2612.5. Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci U S A. 2005. 102:7677–7682.6. Menon U, Jacobs IJ. Recent developments in ovarian cancer screening. Curr Opin Obstet Gynecol. 2000. 12:39–42.7. Chambers AF, Vanderhyden BC. Ovarian cancer biomarkers in urine. Clin Cancer Res. 2006. 12:323–327.8. Goff BA, Muntz HG. Screenig and early diagnosis of ovarian cancer. Womens Health Prim Care. 2005. 8:262–268.9. Ye B, Skates S, Mok SC, Horick NK, Rosenberg HF, Vitonis A, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006. 12:432–441.10. Asai-Sato M, Nagashima Y, Miyagi E, Sato K, Ohta I, Vonderhaar BK, et al. Prolactin inhibits apoptosis of ovarian carcinoma cells induced by serum starvation or cisplatin treatment. Int J Cancer. 2005. 115:539–544.11. Choi JH, Park SH, Leung PC, Choi KC. Expression of leptin receptors and potential effects of leptin on the cell growth and activation of mitogen-activated protein kinases in ovarian cancer cells. J Clin Endocrinol Metab. 2005. 90:207–210.12. Daughaday WH, Rotwein P. Insulin-like growth factors I and II: peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr Rev. 1989. 10:68–91.13. Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001. 1:621–632.14. Hudson JD, Shoaibi MA, Maestro R, Carnero A, Hannon GJ, Beach DH. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999. 190:1375–1382.15. Stattin P, Palmqvist R, Soderberg S, Biessy C, Ardnor B, Hallmans G, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep. 2003. 10:2015–2021.16. Stattin P, Soderberg S, Biessy C, Lenner P, Hallmans G, Kaaks R, et al. Plasma leptin and breast cancer risk: a prospective study in northern Sweden. Breast Cancer Res Treat. 2004. 86:191–196.17. Mantovani G, Maccio A, Mura L, Massa E, Mudu MC, Mulas C, et al. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med. 2000. 78:554–561.18. Hegyi K, Fulop K, Kovacs K, Toth S, Falus A. Leptin-induced signal transduction pathways. Cell Biol Int. 2004. 28:159–169.19. Ruffion A, Al-Sakkaf KA, Brown BL, Eaton CL, Hamdy FC, Dobson PR. The survival effect of prolactin on PC3 prostate cancer cells. Eur Urol. 2003. 43:301–308.20. Peirce SK, Chen WY. Human prolactin and its antagonist, hPRL-G129R, regulate bax and bcl-2 gene expression in human breast cancer cells and transgenic mice. Oncogene. 2004. 23:1248–1255.21. Fedarko NS, Jain A, Karadag A, Van Eman MR, Fisher LW. Elevated serum bone sialoprotein and osteopontin in colon, breast, prostate, and lung cancer. Clin Cancer Res. 2001. 7:4060–4066.22. Conover CA, Hartmann LC, Bradley S, Stalboerger P, Klee GG, Kalli KR, et al. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: the insulin-like growth factor system. Exp Cell Res. 1998. 238:439–449.23. Baron-Hay S, Boyle F, Ferrier A, Scott C. Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res. 2004. 10:1796–1806.24. Brakora KA, Lee H, Yusuf R, Sullivan L, Harris A, Colella T, et al. Utility of osteopontin as a biomarker in recurrent epithelial ovarian cancer. Gynecol Oncol. 2004. 93:361–365.25. But I, Gorisek B. Preoperative value of CA 125 as a reflection of tumor grade in epithelial ovarian cancer. Gynecol Oncol. 1996. 63:166–172.26. Nagele F, Petru E, Medl M, Kainz C, Graf AH, Sevelda P. Preoperative CA 125: an independent prognostic factor in patients with stage I epithelial ovarian cancer. Obstet Gynecol. 1995. 86:259–264.27. Rossi AC, Di Vagno G, Cormio G, Cazzolla A, Stefanelli S, D'Elia E, et al. A retrospective study of preoperative CA 125 levels in 82 patients with ovarian cancer. Arch Gynecol Obstet. 2004. 269:263–265.28. Tuxen MK, Soletormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995. 21:215–245.29. Stratton JF, Pharoah P, Tidy JA, Paterson ME. An analysis of ovarian tumor diameter and survival. Int J Gynecol Cancer. 2000. 10:449–451.30. Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008. 14:1065–1072.31. Kim K, Visintin I, Alvero AB, Mor G. Development and validation of a protein-based signature for the detection of ovarian cancer. Clin Lab Med. 2009. 29:47–55.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- Clinical Evaluation of Serum CEA and CA 19-9 in the Staging of Pancreatic Cancer
- Do we need a better marker for successful ovarian cancer surgery?
- Identification of Biomarkers for Breast Cancer Using Databases
- Role of Tumor Biomarkers in the Surveillance of Hepatocellular Carcinoma