J Gynecol Oncol.
2008 Sep;19(3):162-168. 10.3802/jgo.2008.19.3.162.
Expression of the p16(INK4a) and Ki-67 in relation to the grade of cervical intraepithelial neoplasia and high-risk human papillomavirus infection
- Affiliations
-
- 1Women's Cancer Clinic, Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
- 2Gynecologic Cancer Center, Department of Obstetrics and Gynecology, Kwandong University College of Medicine, Goyang, Korea. jwkim0630@kwandong.ac.kr
- 3Department of Pathology, Kwandong University College of Medicine, Goyang, Korea.
- KMID: 2173396
- DOI: http://doi.org/10.3802/jgo.2008.19.3.162
Abstract
OBJECTIVE
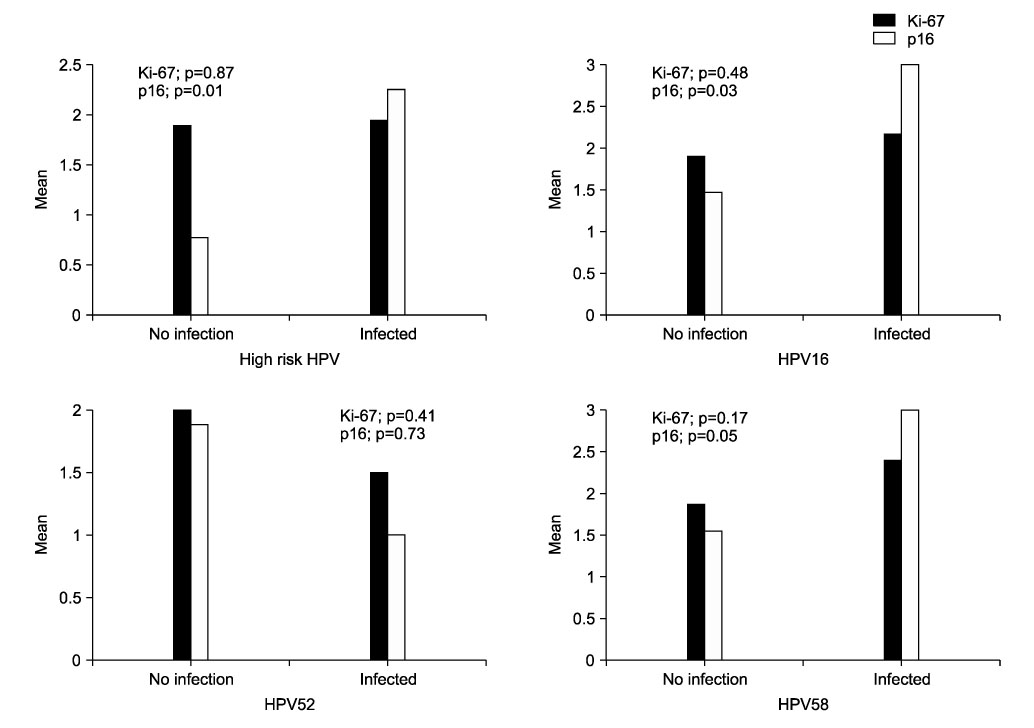
The purposes of this study were to evaluate the expression of p16(INK4a) (referred as to p16) and Ki-67 in cervical intraepithelial neoplasia (CIN), and the correlation between high-risk human papillomavirus (HPV) infection and the above biomarkers. METHODS: We analyzed 31 patients who were diagnosed with CIN at Kwandong University Myongji Hospital from October 2006 to September 2007. CIN specimens (CIN1, 12; CIN2, 6; CIN3, 13) were obtained by colposcopy-directed biopsy (CDB) or loop electrical excision procedure (LEEP). The expressions of p16 and Ki-67 were evaluated by immunohistochemical methods with antibodies to p16 and Ki67. The immunohistochemical staining results were classified into four grades: 0, 1, 2 and 3. HPV genotyping or Hybrid Capture-II test was used to detect high-risk HPV. RESULTS: The expression of p16 (p<0.001) and Ki-67 (p=0.003) were positively associated with CIN grade. p16 expressions increased significantly with high-risk HPV infection (p=0.014), especially HPV type 16 and 58. Ki-67 expression was not related with high-risk HPV. There was positive correlation between the expression of the p16 and Ki-67 (p=0.007). CONCLUSION: CIN grade were positively related to the expression of p16 and Ki-67. p16 expressions of high-risk HPV specimens significantly increased more than Ki-67. Therefore, in the diagnosis of CIN and high-risk HPV infection, p16 can be a useful biomarker.
Keyword
MeSH Terms
Figure
Reference
-
1. zur Hausen H. Human papillomaviruses and their possible role in squamous cell carcinomas. Curr Top Microbiol Immunol. 1977. 78:1–30.2. Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995. 87:796–802.3. Lorincz AT, Reid R, Jenson AB, Greenberg MD, Lancaster W, Kurman RJ. Human papillomavirus infection of the cervix: Relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992. 79:328–337.4. Pfister H. Human papillomaviruses and genital cancer. Adv Cancer Res. 1987. 48:113–147.5. Slebos RJ, Lee MH, Plunkett BS, Kessis TD, Williams BO, Jacks T, et al. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994. 91:5320–5324.6. Nam EJ, Kim YT. Alteration of cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol Cancer. 2008. Feb. 19. [Epub ahead of print].7. Nam EJ, Kim HY, Kim SW, Yoon BS, Kim JH, Kim YT, et al. Relationship between p16INK4a, pRb and high risk HPV infection and recurrence. Korean J Obstet Gynecol. 2006. 49:1437–1445.8. Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991. 65:1053–1061.9. Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989. 243:934–937.10. Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, et al. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994. 264:436–440.11. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.12. Nam EJ, Kim JW, Kim SW, Kim YT, Kim JH, Yoon BS, et al. The expressions of the Rb pathway in cervical intraepithelial neoplasia; predictive and prognostic significance. Gynecol Oncol. 2007. 104:207–211.13. al-Saleh W, Delvenne P, Greimers R, Fridman V, Doyen J, Boniver J. Assessment of Ki-67 antigen immunostaining in squamous intraepithelial lesions of the uterine cervix. Correlation with the histologic grade and human papillomavirus type. Am J Clin Pathol. 1995. 104:154–160.14. Pirog EC, Baergen RN, Soslow RA, Tam D, DeMattia AE, Chen YT, et al. Diagnostic accuracy of cervical low-grade squamous intraepithelial lesions is improved with MIB-1 immunostaining. Am J Surg Pathol. 2002. 26:70–75.15. Isacson C, Kessis TD, Hedrick L, Cho KR. Both cell proliferation and apoptosis increase with lesion grade in cervical neoplasia but do not correlate with human papillomavirus type. Cancer Res. 1996. 56:669–674.16. Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001. 25:884–891.17. Klaes R, Friedrich T, Spitkovsky D, Ridder R, Rudy W, Petry U, et al. Overexpression of p16INK4a as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001. 92:276–284.18. Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16INK4a expression correlates with degree of cervical neoplasia: A comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003. 16:665–673.19. Wang SS, Trunk M, Schiffman M, Herrero R, Sherman ME, Burk RD, et al. Validation of p16INK4a as a marker of oncogenic human papillomavirus infection in cervical biopsies from a population-based cohort in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004. 13:1355–1360.20. Murphy N, Ring M, Heffron CC, King B, Killalea AG, Hughes C, et al. p16INK4a, CDC6, and MCM5: Predictive biomarkers in cervical preinvasive neoplasia and cervical cancer. J Clin Pathol. 2005. 58:525–534.21. Lorenzato M, Caudroy S, Bronner C, Evrard G, Simon M, Durlach A, et al. Cell cycle and/or proliferation markers: What is the best method to discriminate cervical high-grade lesions. Hum Pathol. 2005. 36:1101–1107.22. Sakaguchi M, Fujii Y, Hirabayashi H, Yoon HE, Komoto Y, Oue T, et al. Inversely correlated expression of p16 and Rb protein in non-small cell lung cancers: An immunohistochemical study. Int J Cancer. 1996. 65:442–445.23. Hofmann F, Martelli F, Livingston DM, Wang Z. The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev. 1996. 10:2949–2959.24. Hateboer G, Kerkhoven RM, Shvarts A, Bernards R, Beijersbergen RL. Degradation of E2F by the ubiquitin-proteasome pathway: Regulation by retinoblastoma family proteins and adenovirus transforming proteins. Genes Dev. 1996. 10:2960–2970.25. Song SH, Park HM, Eom DW, Lee JK, Lee NW, Kim AR, et al. The expression of p16INK4a and Ki-67 in relation to high-risk human papilloma viral load and residual disease after conization with positive margins. Int J Gynecol Cancer. 2007. 17:858–867.26. Hwang HS, Park M, Lee SY, Kwon KH, Pang MG. Distribution and prevalence of human papillomavirus genotypes in routine pap smear of 2,470 Korean women determined by DNA chip. Cancer Epidemiol Biomarkers Prev. 2004. 13:2153–2156.27. Gage JR, Meyers C, Wettstein FO. The E7 proteins of the non-oncogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J Virol. 1990. 64:723–730.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between p16(INK4a), pRb and high risk HPV infection and recurrence
- Studies on the Expression of the p16 (INK4A), p53, and Ki-67 Labeling Index in Inflammatory and Neoplastic Diseases of the Uterine Cervix
- Diagnosis of Cervical Neoplasia Using Immunohistochemical Staining of p16(INK4A)
- The prognostic significance of p16, Ki-67, p63, and CK17 expression determined by immunohistochemical staining in cervical intraepithelial neoplasia 1
- Overexpression of p16(INK4A) as a biomarker for ASCUS in ThinPrep(TM) smear