Blood Res.
2014 Jun;49(2):115-119. 10.5045/br.2014.49.2.115.
Abbreviated chemotherapy for limited-stage diffuse large B-cell lymphoma after complete resection
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csuh@amc.seoul.kr
- 2Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2172807
- DOI: http://doi.org/10.5045/br.2014.49.2.115
Abstract
- BACKGROUND
Abbreviated chemotherapy followed by radiotherapy or full cycles of chemotherapy is recommended as a standard treatment for limited-stage (LS) diffuse large B-cell lymphoma (DLBCL). After complete resection of tumors, however, Burkitt and childhood B-cell Non-Hodgkin lymphoma show favorable outcomes, even after abbreviated chemotherapy of only 2 or 3 cycles. We investigated the effectiveness of abbreviated chemotherapy in patients with LS DLBCL after complete tumor resection.
METHODS
We retrospectively reviewed 18 patients with LS DLBCL who underwent complete tumor resection followed by either 3 or 4 cycles of chemotherapy between March 2002 and May 2010.
RESULTS
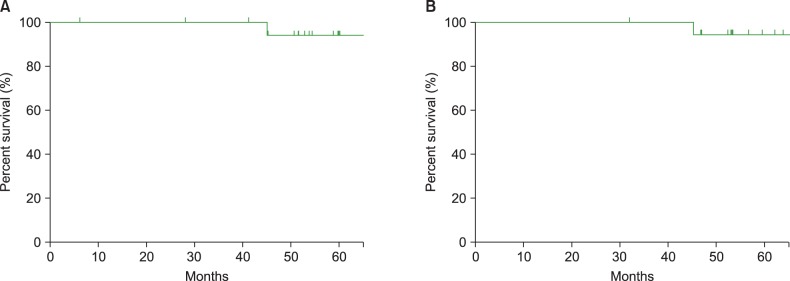
With a median follow-up period of 57.9 months (range, 31.8-130.2 months), no patients experienced disease relapse or progression; however, 1 patient experienced secondary acute myeloid leukemia during follow-up. The 5-year progression-free survival rate and overall survival rate were 93.3% and 94.1%, respectively.
CONCLUSION
These results warrant further investigation into abbreviated chemotherapy as an alternative treatment for patients who have undergone complete resection of LS DLBCL.
MeSH Terms
Figure
Reference
-
1. Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N Engl J Med. 1998; 339:21–26. PMID: 9647875.
Article2. Hong J, Kim AJ, Park JS, et al. Additional rituximab-CHOP (R-CHOP) versus involved-field radiotherapy after a brief course of R-CHOP in limited, non-bulky diffuse large B-cell lymphoma: a retrospective analysis. Korean J Hematol. 2010; 45:253–259. PMID: 21253427.
Article3. Kim SJ, Kang HJ, Kim JS, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 2011; 117:1958–1965. PMID: 21148334.
Article4. Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkins lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001; 19:3861–3873. PMID: 11559724.
Article5. Rawls RA, Vega KJ, Trotman BW. Small Bowel Lymphoma. Curr Treat Options Gastroenterol. 2003; 6:27–34. PMID: 12521569.
Article6. Diviné M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol. 2005; 16:1928–1935. PMID: 16284057.
Article7. Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt's lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002; 13:1264–1274. PMID: 12181251.
Article8. Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Münster Group Trial NHL-BFM 90. Blood. 1999; 94:3294–3306. PMID: 10552938.9. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114:937–951. PMID: 19357394.
Article10. Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25:579–586. PMID: 17242396.11. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008; 26:2258–2263. PMID: 18413640.
Article12. Pfreundschuh M, Trumper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–391. PMID: 16648042.13. Pfreundschuh M, Kuhnt E, Trumper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011; 12:1013–1022. PMID: 21940214.
Article14. Ziepert M, Hasenclever D, Kuhnt E, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin Oncol. 2010; 28:2373–2380. PMID: 20385988.15. Bonnet C, Fillet G, Mounier N, et al. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2007; 25:787–792. PMID: 17228021.
Article16. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242. PMID: 11807147.
Article17. Pirani M, Marcheselli R, Marcheselli L, Bari A, Federico M, Sacchi S. Risk for second malignancies in non-Hodgkin's lymphoma survivors: a meta-analysis. Ann Oncol. 2011; 22:1845–1858. PMID: 21310758.
Article18. Praga C, Beretta G, Vigo PL, et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep. 1979; 63:827–834. PMID: 455324.19. Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979; 91:710–717. PMID: 496103.20. Bristow MR, Mason JW, Billingham ME, Daniels JR. Dose-effect and structure-function relationships in doxorubicin cardiomyopathy. Am Heart J. 1981; 102:709–718. PMID: 7282516.
Article21. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003; 97:2869–2879. PMID: 12767102.22. Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996; 125:47–58. PMID: 8644988.
Article23. Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes-a report from the Children's Oncology Group. J Clin Oncol. 2012; 30:1415–1421. PMID: 22124095.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffuse large B-cell lymphoma presenting with cholecystitis-like symptoms
- Relapse of Ocular Lymphoma following Primary Testicular Diffuse Large B-cell Lymphoma
- Two Cases of Primary Esophageal Diffuse Large B Cell Lymphoma: Therapeutic Considerations and a Literature Review
- A Case of Solitary Relapsed Diffuse Large B-cell Lymphoma of the Gallbladder
- Therapeutic Comparison of Chemotherapy and Surgery for Early Stage Diffuse Large B-cell Gastric Lymphoma