Blood Res.
2016 Mar;51(1):37-43. 10.5045/br.2016.51.1.37.
Long-term course of anti-factor VIII antibody in patients with hemophilia A at a single center
- Affiliations
-
- 1Korea Hemophilia Foundation, Seoul, Korea. gowho@hanmail.net
- KMID: 2172743
- DOI: http://doi.org/10.5045/br.2016.51.1.37
Abstract
- BACKGROUND
Immune tolerance induction (ITI) can reduce inhibitors against factor VIII concentrates by 70-80%. In this study, we elucidated the characteristics of inhibitors and attempted to determine the proper indications and timing for ITI.
METHODS
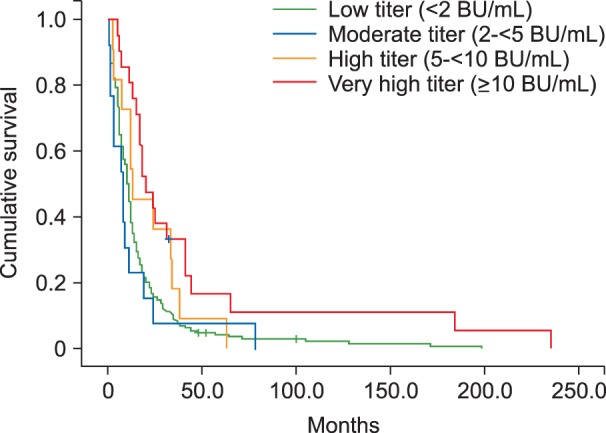
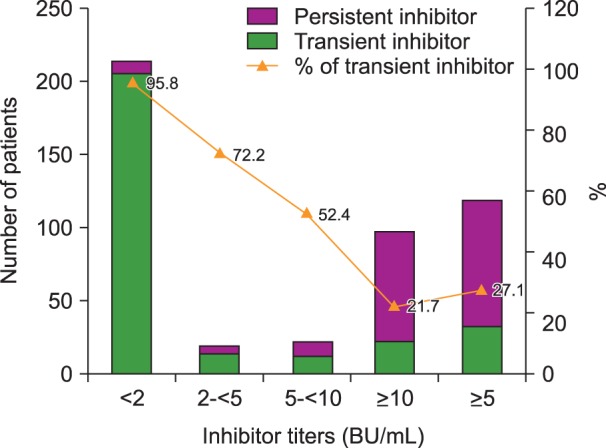
Subjects included hemophilia A patients registered at the Korea Hemophilia Foundation from 1991 through 2014. Inhibitors were classified as persistent and transient. Patients were classified into groups according to peak inhibitor titer: low (<2 BU/mL), moderate (2 to <5 BU/mL), high (5 to <10 BU/mL), and very high titer (≥10 BU/mL).
RESULTS
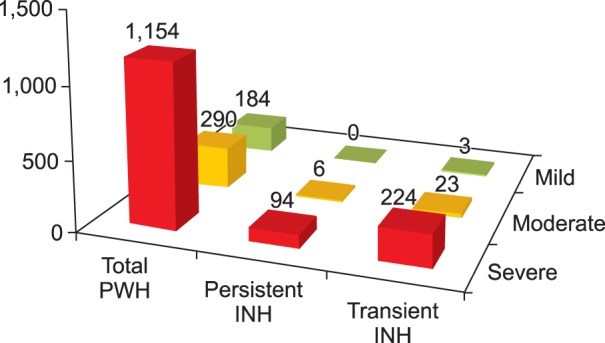
Overall, 350 (21.4%) of 1,634 hemophilia A patients developed inhibitors at least once. Of these, 100 (6.1%) and 250 (15.3%) patients developed persistent and transient inhibitors, respectively. For transient inhibitors, the median peak titer was 1.0 BU/mL, persistent for median of 11.0 months (10.0, 8.0, 13.0, and 19.0 months in the low, moderate, high, and very high titer transient inhibitor groups, respectively). Overall, 95.8% (215), 72.2% (17), 52.4% (21), and 21.7% (97) of patients in the low, moderate, high, and very high titer groups became inhibitor-negative spontaneously, without ITI.
CONCLUSION
Given the spontaneous disappearance of inhibitors and high cost of ITI, it is worthwhile to postpone ITI for 11 months unless the peak inhibitor titer is greater than 10 BU/mL.
Keyword
Figure
Reference
-
1. Andersen BR, Terry WD. Gamma G4-globulin antibody causing inhibition of clotting factor VIII. Nature. 1968; 217:174–175.
Article2. Brackmann HH, Schwaab R, Effenberger W, Hess L, Hanfland P, Oldenburg J. Antibodies to factor VIII in hemophilia A patients. Vox Sang. 2000; 78(Suppl 2):187–190. PMID: 10938950.3. Colowick AB, Bohn RL, Avorn J, Ewenstein BM. Immune tolerance induction in hemophilia patients with inhibitors: costly can be cheaper. Blood. 2000; 96:1698–1702. PMID: 10961866.
Article4. Yoo KY, Kim SK, Kwon SS, et al. Life expectancy of Korean haemophiliacs, 1991-2012. Haemophilia. 2014; 20:e356–e358. PMID: 24948408.
Article5. Brackmann HH, Wallny T. Immune tolerance: high-dose regimen. In : Rodriquez-Merchan EC, Lee CA, editors. Inhibitors in patients with haemophilia. Oxford, UK: Blackwell Science;2002. p. 45–48.6. Darby SC, Keeling DM, Spooner RJ, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977-99. J Thromb Haemost. 2004; 2:1047–1054. PMID: 15219185.
Article7. Warrier I, Lusher JM. Development of anaphylactic shock in haemophilia B patients with inhibitors. Blood Coagul Fibrinolysis. 1998; 9(Suppl 1):S125–S128. PMID: 9819043.8. Iorio A. Epidemiology of inhibitors in hemophilia. In : Lee CA, Berntorp EE, Hoots WK, editors. Textbook of hemophilia. 3rd ed. Oxford, UK: Wiley-Blackwell;2014. p. 53–58.9. van der Bom JG, ter Avest P, Van den Berg HM, Psaty BM, Weiss NS. Assessment of incidence of inhibitors in patients with haemophilia. Haemophilia. 2009; 15:707–711. PMID: 19432923.
Article10. Kroner BL. Comparison of the international immune tolerance registry and the North American immune tolerance registry. Vox Sang. 1999; 77(Suppl 1):33–37. PMID: 10529685.
Article11. Caram C, de Souza RG, de Sousa JC, et al. The long-term course of factor VIII inhibitors in patients with congenital haemophilia A without immune tolerance induction. Thromb Haemost. 2011; 105:59–65. PMID: 21057702.
Article12. Hay CR, Brown S, Collins PW, Keeling DM, Liesner R. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophilia Centre Doctors Organisation. Br J Haematol. 2006; 133:591–605. PMID: 16704433.
Article13. Hoyer LW. The incidence of factor VIII inhibitors in patients with severe hemophilia A. Adv Exp Med Biol. 1995; 386:35–45. PMID: 8851013.
Article14. Ören H, Yaprak I, İrken G. Factor VIII inhibitors in patients with hemophilia A. Acta Haematol. 1999; 102:42–46. PMID: 10473887.
Article15. Mauser-Bunschoten EP, van der Bom JG, Bongers M, et al. Purity of factor VIII product and incidence of inhibitors in previously untreated patients with haemophilia A. Haemophilia. 2001; 7:364–368. PMID: 11442640.
Article16. Mauser-Bunschoten EP, Nieuwenhuis HK, Roosendaal G, van den Berg HM. Low-dose immune tolerance induction in hemophilia A patients with inhibitors. Blood. 1995; 86:983–988. PMID: 7620189.
Article17. Feinstein DI. Inhibitors in hemophilia. In : Hoffman R, Benz EJ, Shattil SJ, editors. Hematology: Basic principles and practice. 4th ed. Philadelphia, PA: Elsevier;2005. p. 2071–2080.18. DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007; 13(Suppl 1):1–22. PMID: 17593277.
Article19. Feinstein DI. Inhibitors in hemophilia. In : Hoffman R, Benz EJ, Shattil SJ, editors. Hematology: Basic principles and practice. 3rd ed. Philadelphia, PA: Churchill Livingstone;2000. p. 1904–1911.20. Mauser-Bunschoten EP, Rosendaal FR, Nieuwenhuis HK, Roosendaal G, Briët E, van den Berg HM. Clinical course of factor VIII inhibitors developed after exposure to a pasteurised Dutch concentrate compared to classic inhibitors in hemophilia A. Thromb Haemost. 1994; 71:703–706. PMID: 7974335.
Article21. Sharifian R, Hosseini M, Safai R, et al. Prevalence of inhibitors in a population of 1280 hemophilia A patients in Iran. Acta Medica Iranica. 2003; 41:66–68.22. Rossetti LC, Szurkalo I, Radic CP, et al. Factor VIII genotype characterization of haemophilia A affected patients with transient and permanent inhibitors: a comprehensive Argentine study of inhibitor risks. Haemophilia. 2013; 19:511–518. PMID: 23534532.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Autoplex Treatment in a Hemophilia A Patient with High Titer of Anticoagulant FVIII Antibody
- Factor VIII Gene Inversions in Korean Patients with Severe Hemophilia A and its Application to Carrier Detection
- Iliacus Hematoma with Femoral Neuropathy in Hemophilia: A Case report
- Cranial Hemophilic Pseudotumor: Case Report
- Genetic Risk Factors of Hemophilia A