Ann Dermatol.
2016 Apr;28(2):179-185. 10.5021/ad.2016.28.2.179.
Potential Role of S100A8 in Cutaneous Squamous Cell Carcinoma Differentiation
- Affiliations
-
- 1Department of Dermatology and Research Institute for Medical Sciences, Chungnam National University School of Medicine, Daejeon, Korea. resina20@cnu.ac.kr
- 2Department of Anatomy, Chungnam National University School of Medicine, Daejeon, Korea.
- 3Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 2171374
- DOI: http://doi.org/10.5021/ad.2016.28.2.179
Abstract
- BACKGROUND
S100A8 is differentially expressed in various cell types and is associated with a number of malignant disorders. S100A8 may affect tumor biology. However, its role in cutaneous squamous cell carcinoma (SCC) is not well established.
OBJECTIVE
This study aims to investigate the relationship between S100A8 and cutaneous SCC development.
METHODS
We performed immunohistochemical staining to detect S100A8 expression in facial skin specimens of premalignant actinic keratosis (AK), malignant SCC, and normal tissues. In addition, we utilized postconfluence and high calcium-induced differentiation in a culture system model. Furthermore, we constructed a recombinant adenovirus expressing GFP-tagged S100A8 to investigate the role of S100A8 in SCC cell differentiation.
RESULTS
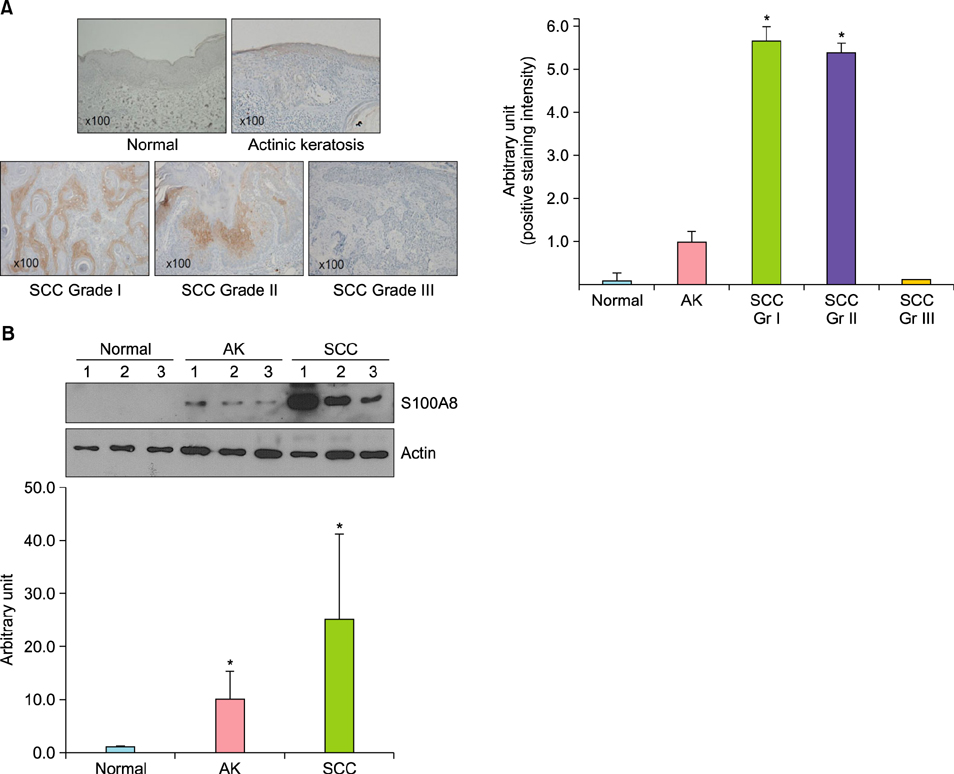
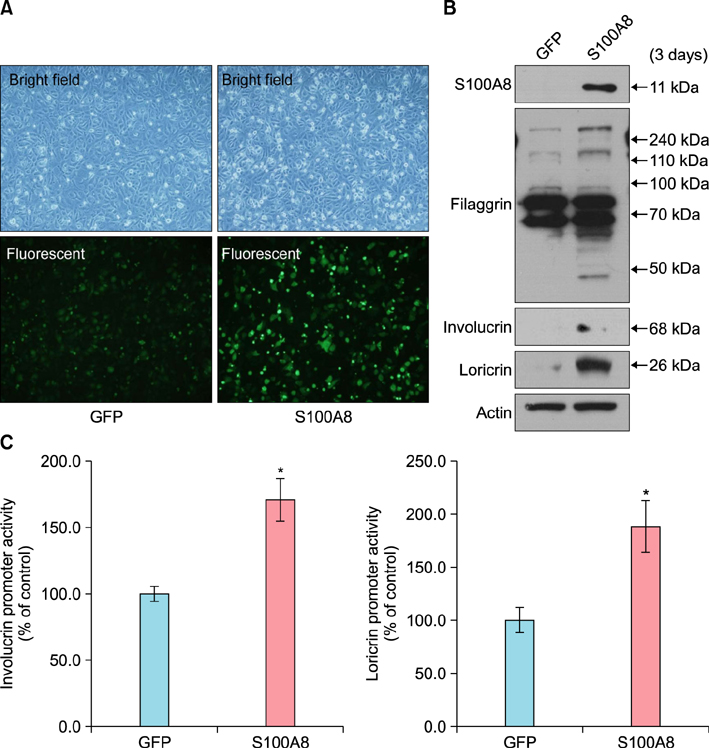
S100A8 was significantly overexpressed in human cutaneous SCC compared to that in normal and AK tissues. S100A8 was gradually upregulated in SCC cells in a post-confluence-induced differentiation model. Overexpression of S100A8 in SCC cells induced by adenoviral transduction led to increased expression levels of differentiation markers, such as loricrin, involucrin, and filaggrin. S100A8 overexpression also increased loricrin and involucrin luciferase activity.
CONCLUSION
S100A8 regulates cutaneous SCC differentiation and induces well-differentiated SCC formation in skin.
MeSH Terms
Figure
Cited by 1 articles
-
β-Catenin Regulates the Expression of cAMP Response Element-Binding Protein 1 in Squamous Cell Carcinoma Cells
Soo-Yeon Kim, Jin-Hyup Lee, Kyung-Cheol Sohn, Myung Im, Young Lee, Young-Joon Seo, Jeung-Hoon Lee, Chang-Deok Kim
Ann Dermatol. 2018;30(1):119-122. doi: 10.5021/ad.2018.30.1.119.
Reference
-
1. Grossman D, Leffel DJ. Squamous cell carcinoma. In : Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill;2012. p. 1283–1294.2. Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). J Am Acad Dermatol. 1992; 26:467–484.
Article3. Feldman SR, Fleischer AB Jr. Progression of actinic keratosis to squamous cell carcinoma revisited: clinical and treatment implications. Cutis. 2011; 87:201–207.4. Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol. 2003; 170:3233–3242.
Article5. Roth J, Vogl T, Sorg C, Sunderkötter C. Phagocyte-specific S100 proteins: a novel group of proinflammatory molecules. Trends Immunol. 2003; 24:155–158.
Article6. Kerkhoff C, Voss A, Scholzen TE, Averill MM, Zänker KS, Bornfeldt KE. Novel insights into the role of S100A8/A9 in skin biology. Exp Dermatol. 2012; 21:822–826.
Article7. Petersen S, Aninat-Meyer M, Schlüns K, Gellert K, Dietel M, Petersen I. Chromosomal alterations in the clonal evolution to the metastatic stage of squamous cell carcinomas of the lung. Br J Cancer. 2000; 82:65–73.
Article8. Jee KJ, Gong G, Ahn SH, Park JM, Knuutila S. Gain in 1q is a common abnormality in phyllodes tumours of the breast. Anal Cell Pathol. 2003; 25:89–93.
Article9. Knösel T, Petersen S, Schwabe H, Schlüns K, Stein U, Schlag PM, et al. Incidence of chromosomal imbalances in advanced colorectal carcinomas and their metastases. Virchows Arch. 2002; 440:187–194.
Article10. Sy SM, Wong N, Lai PB, To KF, Johnson PJ. Regional over-representations on chromosomes 1q, 3q and 7q in the progression of hepatitis B virus-related hepatocellular carcinoma. Mod Pathol. 2005; 18:686–692.
Article11. Yong HY, Moon A. Roles of calcium-binding proteins, S100A8 and S100A9, in invasive phenotype of human gastric cancer cells. Arch Pharm Res. 2007; 30:75–81.
Article12. Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D. S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res. 2006; 312:184–197.
Article13. Stulík J, Koupilova K, Osterreicher J, Knízek J, Macela A, Bures J, et al. Protein abundance alterations in matched sets of macroscopically normal colon mucosa and colorectal carcinoma. Electrophoresis. 1999; 20:3638–3646.
Article14. Stulík J, Osterreicher J, Koupilová K, Knízek , Macela A, Bures J, et al. The analysis of S100A9 and S100A8 expression in matched sets of macroscopically normal colon mucosa and colorectal carcinoma: the S100A9 and S100A8 positive cells underlie and invade tumor mass. Electrophoresis. 1999; 20:1047–1054.
Article15. Luo A, Kong J, Hu G, Liew CC, Xiong M, Wang X, et al. Discovery of Ca2+-relevant and differentiation-associated genes downregulated in esophageal squamous cell carcinoma using cDNA microarray. Oncogene. 2004; 23:1291–1299.
Article16. Ito Y, Arai K, Nozawa R, Yoshida H, Hirokawa M, Fukushima M, et al. S100A8 and S100A9 expression is a crucial factor for dedifferentiation in thyroid carcinoma. Anticancer Res. 2009; 29:4157–4161.17. Arai K, Takano S, Teratani T, Ito Y, Yamada T, Nozawa R. S100A8 and S100A9 overexpression is associated with poor pathological parameters in invasive ductal carcinoma of the breast. Curr Cancer Drug Targets. 2008; 8:243–252.
Article18. Duan L, Wu R, Ye L, Wang H, Yang X, Zhang Y, et al. S100A8 and S100A9 are associated with colorectal carcinoma progression and contribute to colorectal carcinoma cell survival and migration via Wnt/β-catenin pathway. PLoS One. 2013; 8:e62092.
Article19. Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013; 68:957–966.
Article20. Brantsch KD, Meisner C, Schönfisch B, Trilling B, Wehner-Caroli J, Röcken M, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008; 9:713–720.
Article21. Mourouzis C, Boynton A, Grant J, Umar T, Wilson A, Macpheson D, et al. Cutaneous head and neck SCCs and risk of nodal metastasis - UK experience. J Craniomaxillofac Surg. 2009; 37:443–447.
Article22. Cassarino DS, Derienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification--part two. J Cutan Pathol. 2006; 33:261–279.
Article23. Sereno M, Esteban IR, Zambrana F, Merino M, Gómez-Raposo C, López-Gómez M, et al. Squamous-cell carcinoma of the lungs: is it really so different? Crit Rev Oncol Hematol. 2012; 84:327–339.
Article24. Ambe P, Shadouh S, Granetzny S, Köhler L. Squamous cell carcinoma of the colon. A rare histological entity. Chirurg. 2011; 82:1116–1119.25. Dyson T, Draganov PV. Squamous cell cancer of the rectum. World J Gastroenterol. 2009; 15:4380–4386.
Article26. Watanabe S, Ichikawa E, Takahashi H, Otsuka F. Changes of cytokeratin and involucrin expression in squamous cell carcinomas of the skin during progression to malignancy. Br J Dermatol. 1995; 132:730–739.
Article27. Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, et al. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009; 625:73–83.
Article28. Srikrishna G. S100A8 and S100A9: new insights into their roles in malignancy. J Innate Immun. 2012; 4:31–40.
Article29. Choi JH, Shin NR, Moon HJ, Kwon CH, Kim GH, Song GA, et al. Identification of S100A8 and S100A9 as negative regulators for lymph node metastasis of gastric adenocarcinoma. Histol Histopathol. 2012; 27:1439–1448.30. Hummerich L, Müller R, Hess J, Kokocinski F, Hahn M, Fürstenberger G, et al. Identification of novel tumourassociated genes differentially expressed in the process of squamous cell cancer development. Oncogene. 2006; 25:111–121.
Article31. Voss A, Bode G, Sopalla C, Benedyk M, Varga G, Böhm M, et al. Expression of S100A8/A9 in HaCaT keratinocytes alters the rate of cell proliferation and differentiation. FEBS Lett. 2011; 585:440–446.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cutaneous Metastasis of Esophageal Squamous Cell Carcinoma Mimicking Benign Soft Tissue Tumor
- A Case of Metastatic Cutaneous Squamous Cell Carcinoma Arising in Chronic Osteomyelitic Focus
- Pseudoangiosarcomatous Squamous Cell Carcinoma of the Face
- Merkel Cell Carcinoma
- Prognostic Role of S100A8 and S100A9 Protein Expressions in Non-small Cell Carcinoma of the Lung