Ewha Med J.
2015 Mar;38(1):46-49. 10.12771/emj.2015.38.1.46.
Ovarian Metastasis from Non-Small Cell Lung Cancer Responding to Erlotinib
- Affiliations
-
- 1Department of Internal Medicine, Konyang University College of Medicine, Daejeon, Korea. dycho@kyuh.ac.kr
- KMID: 2171329
- DOI: http://doi.org/10.12771/emj.2015.38.1.46
Abstract
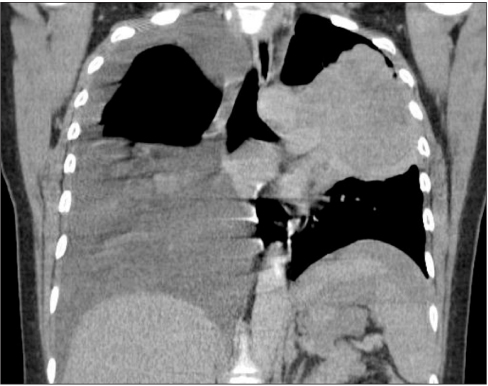
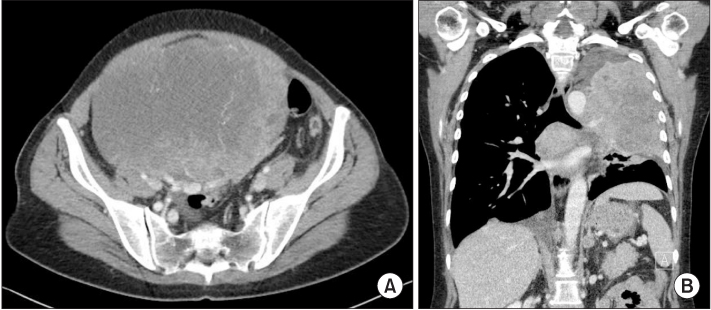
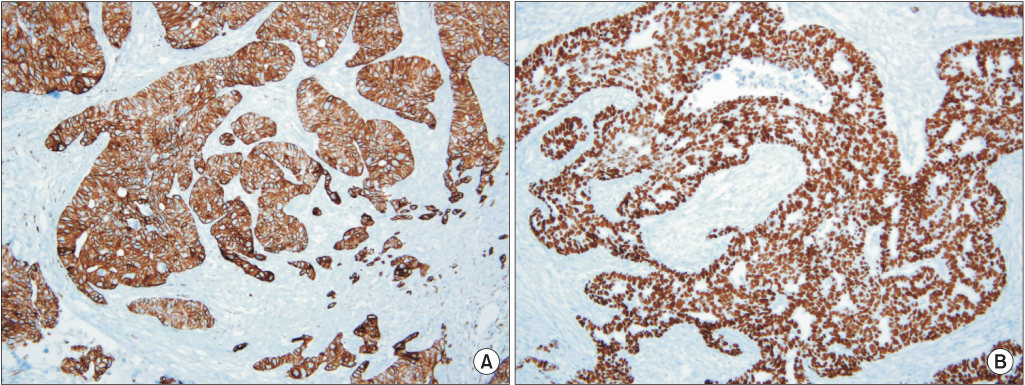
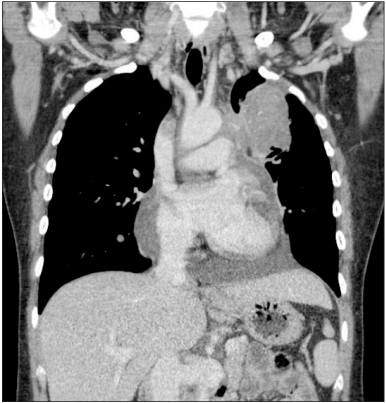
- Ovarian cancer is generally primary cancer and less frequently originates from metastasis from non-gynecological cancer. Ovarian metastasis from lung cancer represents only 2~4% of all ovarian metastatic cancers. We report a case of ovarian metastasis of non-small cell lung cancer with epidermal growth factor receptor mutation. The patient underwent cytoreductive surgery for the ovarian mass and erlotinib therapy for the metastatic lung cancer. Erlotinib therapy markedly decreased the size of lung mass.
Keyword
MeSH Terms
Figure
Reference
-
1. Hart W. Diagnostic challenge of secondary (metastatic) ovarian tumors simulating primary endometrioid and mucinous neoplasms. Pathol Int. 2005; 55:231–243.2. Horn L, Pao W, Johnson D. Neoplasm of the Lung. In : Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw Hill;2012. p. 745–750.3. Schiller J, Harrington D, Belani C, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98.4. Lynch T, Bell D, Sordella R, Gurubhagavatula S, Okimoto R, Brannigan B, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefetinib. N Engl J Med. 2004; 350:2129–2139.5. Fader A, Rose P. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007; 25:2873–2883.6. Jung YE, Lee JW, Kim BG, Bae DS. Ovarian metastasis from pulmonary adenocarcinoma. Obstet Gynecol Sci. 2013; 56:341–344.7. Varadhachary G, Abbruzzese J. Carcinoma of Unknown Primary. In : Longo D, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw Hill;2012. p. 822–823.8. Pfister D, Johnson D, Azzoli C, Sause W, Smith T, Baker S, et al. American society of clinical oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330–353.9. Hirsch F, Varella-Garcia M, Bunn P Jr, Franklin W, Dziadziuszko R, Thatcher N, et al. Molecular predictors of outcome with gefetinib in a phase III placebo-controlled study in advanced nonsmall-cell lung cancer. J Clin Oncol. 2006; 24:5034–5042.10. Sequist L, Bell D, Lynch T, Haber D. Molecular predictors of response to epidermal growth factor receptor antagonists in nonsmall-cell lung cancer. J Clin Oncol. 2007; 25:587–595.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Erlotinib-Induced Interstitial Lung Disease on Non-Small Cell Lung Cancer
- Erlotinib HCL (Tarceva(R)) Induced Radiation Recall Dermatitis
- Bowel Perforation after Erlotinib Treatment in a Patient with Non-Small Cell Lung Cancer
- Two Cases of Cutaneous Metastasis from Small Cell Lung Cancer
- Ovarian metastasis from pulmonary adenocarcinoma