Infect Chemother.
2014 Mar;46(1):21-29. 10.3947/ic.2014.46.1.21.
Underestimation of the Calculated Area Under the Concentration-Time Curve Based on Serum Creatinine for Vancomycin Dosing
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. imfell@yuhs.ac
- 2Therapeutic Drug Monitoring Team, Gangnam Severance Hospital, Seoul, Korea.
- KMID: 2170456
- DOI: http://doi.org/10.3947/ic.2014.46.1.21
Abstract
- BACKGROUND
The ratio of the steady-state 24-hour area under the concentration-time curve (ssAUC24) to the MIC (AUC24/MIC) for vancomycin has been recommended as the preferred pharmacodynamic index. The aim of this study was to assess whether the calculated AUC24 (cAUC24) using the creatinine clearance (CLcr) differs from the ssAUC24 based on the individual pharmacokinetic data estimated by a commercial software.
MATERIALS AND METHODS
The cAUC24 was compared with the ssAUC24 with respect to age, body mass index, and trough concentration of vancomycin and the results were expressed as median and interquartile ranges. A correlation between the cAUC24 and ssAUC24 and the trough concentration of vancomycin was evaluated. The probability of reaching an AUC24/MIC of 400 or higher was compared between the cAUC24 and ssAUC24 for different MICs of vancomycin and different daily doses by simulation in a subgroup with a trough concentration of 10 mg/L and higher.
RESULTS
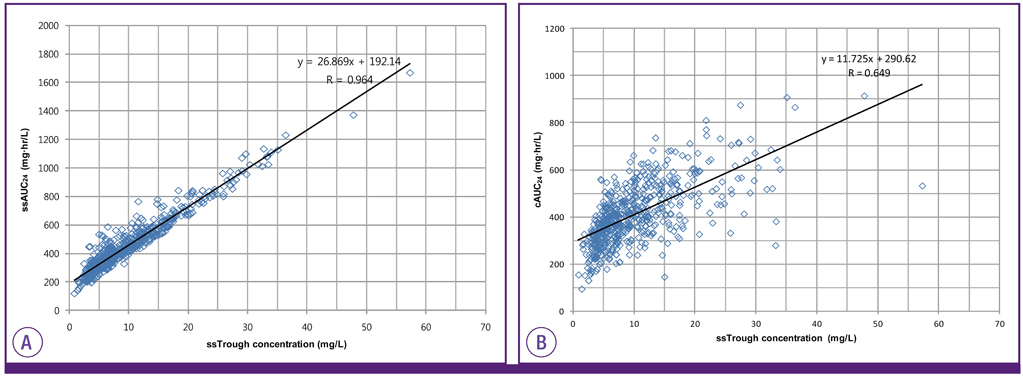
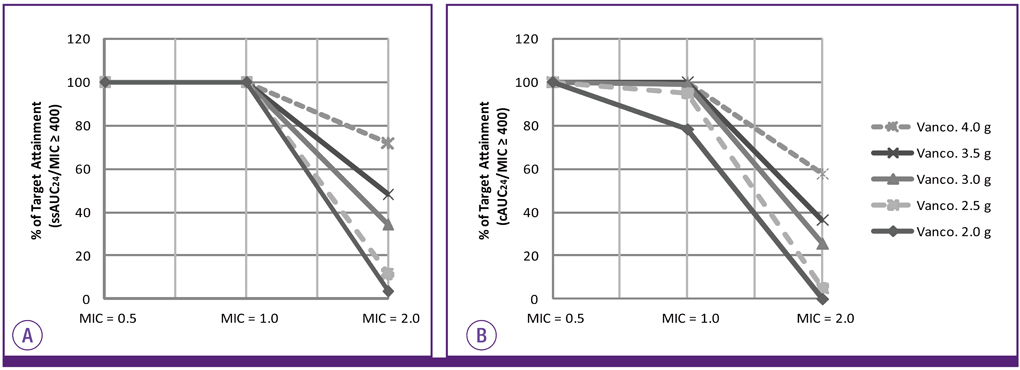
The cAUC24 was significantly lower than the ssAUC24 (392.38 vs. 418.32 mg.hr/L, P < 0.0001) and correlated weakly with the trough concentration (r = 0.649 vs. r = 0.964). Assuming a MIC of 1.0 mg/L, the probability of reaching the value of 400 or higher was 77.5% for the cAUC24/MIC and 100% for the ssAUC24/MIC in patients with a trough concentration of 10 mg/L and higher. If the MIC increased to 2.0 mg/L, the probability was 57.7% for the cAUC24/MIC and 71.8% for the ssAUC24/MIC at a daily vancomycin dose of 4,000 mg.
CONCLUSIONS
The cAUC24 using the calculated CLcr is usually underestimated compared with the ssAUC24 based on individual pharmacokinetic data. Therefore, to obtain a more accurate AUC24, therapeutic monitoring of vancomycin rather than a simple calculation based on the CLcr should be performed, and a more accurate biomarker for renal function is needed.
MeSH Terms
Figure
Cited by 1 articles
-
Overestimation of Vancomycin Clearance by the Linear Regression Formula in Rodvold's Report: Why?
Dong-Seok Yim
Infect Chemother. 2014;46(1):62-63. doi: 10.3947/ic.2014.46.1.62.
Reference
-
1. Helgason KO, Thomason AH, Ferguson C. A review of vancomycin therapeutic drug monitoring recommendations in Scotland. J Antimicrob Chemother. 2008; 61:1398–1399.
Article2. Thomson AH, Staatz CE, Tobin CM, Gall M, Lovering AM. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J Antimicrob Chemother. 2009; 63:1050–1057.
Article3. Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004; 43:925–942.
Article4. Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST, Kollef MH. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. Chest. 2006; 130:947–955.
Article5. Fernández de Gatta Mdel M, Santos Buelga D, Sánchez Navarro A, Dominguez-Gil A, García MJ. Vancomycin dosage optimization in patients with malignant haematological disease by pharmacokinetic/pharmacodynamics analysis. Clin Pharmacokinet. 2009; 48:273–280.6. Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. Vancomycin: we can't get there from here. Clin Infect Dis. 2011; 52:969–974.
Article7. Rodvold KA, Blum RA, Fischer JH, Zokufa HZ, Rotschafer JC, Crossley KB, Riff LJ. Vancomycin pharmacokinetics in patients with various degrees of renal function. Antimicrob Agents Chemother. 1988; 32:848–852.
Article8. Kollef MH. Limitations of vancomycin in the management of resistant Staphylococcal infections. Clin Infect Dis. 2007; 45:Suppl 3. S191–S195.
Article9. Soriano A, Marco F, Martinez JA, Pisos E, Almela M, Dimova VP, Alamo D, Ortega M, Lopez J, Mensa J. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008; 46:193–200.
Article10. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976; 16:31–41.
Article11. Jin SJ, Bae SC, Kim HW, Kim HK, Na EJ, Ahn BS, Choi JY, Kim CO, Kim JM, Song YG. Evaluation of the initial dose of vancomycin using serum cystatin C as a marker in elderly patients. Infect Chemother. 2009; 41:224–229.
Article12. Chung JY, Jin SJ, Yoon JH, Song YG. Serum cystatin C is a major predictor of vancomycin clearance in a population pharmacokinetic analysis of patients with normal serum creatinine concentrations. J Korean Med Sci. 2013; 28:48–54.
Article13. Chung J, Oh JM, Cho EM, Jang HJ, Hong SB, Lim CM, Koh YS. Optimal dose of vancomycin for treating methicillin-resistant Staphylococcus aureus pneumonia in critically ill patients. Anaesth Intensive Care. 2011; 39:1030–1037.
Article14. Song YG, Kim HK, Roe EK, Lee SY, Ahn BS, Kim JH, Park MS, Yoon HJ, Kim JM. Therapeutic drug monitoring of vancomycin in Korean patients. Infect Chemother. 2004; 36:311–318.15. Wie SH, Kim SI, Kim YR, Bae SM, Hong KJ, Ra HO, Kang MW. Therapeutic drug monitoring of vancomycin. Korean J Infect Dis. 2000; 32:141–147.16. Okamoto G, Sakamoto T, Kimura M, Ukishima Y, Sonoda A, Mori N, Kato Y, Maeda T, Kagawa Y. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin Biochem. 2007; 40:485–490.
Article17. Pöge U, Gerhardt T, Stoffel-Wagner B, Klehr HU, Sauerbruch T, Woitas RP. Calculation of glomerular filtration rate based on cystatin C in cirrhotic patients. Nephrol Dial Transplant. 2006; 21:660–664.
Article18. Hermida J, Tutor JC. Serum cystatin C for the prediction of glomerular filtration rate with regard to the dose adjustment of amikacin, gentamicin, tobramycin, and vancomycin. Ther Drug Monit. 2006; 28:326–331.
Article19. Tanaka A, Aiba T, Otsuka T, Suemaru K, Nishimiya T, Inoue T, Murase M, Kurosaki Y, Araki H. Population pharmacokinetic analysis of vancomycin using serum cystatin C as a marker of renal function. Antimicrob Agents Chemother. 2010; 54:778–782.
Article20. Karam CM, McKinnon PS, Neuhauser MM, Rybak MJ. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy. 1999; 19:257–266.
Article21. Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001-05. J Antimicrob Chemother. 2007; 60:788–794.
Article22. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006; 42:Suppl 1. S35–S39.
Article23. Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009; 66:82–98.
Article24. Mohr JF, Murray BE. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2007; 44:1536–1542.
Article25. del Mar Fernández de Gatta Garcia M, Revilla N, Calvo MV, Domínguez-Gil A, Sánchez Navarro A. Pharmacokinetic/pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med. 2006; 33:279–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of a model to predict vancomycin serum concentration during continuous infusion of vancomycin in critically ill pediatric patients
- Evaluation of the Effect of Initial dose of Vancomycin using Serum Cystatin C as a Marker in Elderly Patients
- Comparison of Trough-Based and Area Under the Curve-Based Therapeutic Drug Monitoring of Vancomycin: An In Silico Study
- Evaluation of Vancomycin Area Under the Concentration–Time Curve Predictive Performance Using Bayesian Modeling Software With and Without Peak Concentration: An Academic Hospital Experience for Adult Patients Without Renal Impairment
- Usefulness of serum cystatin C to determine the dose of vancomycin in neonate