Infect Chemother.
2013 Sep;45(3):308-314. 10.3947/ic.2013.45.3.308.
Predictive Performance of Serum Procalcitonin for the Diagnosis of Bacterial Meningitis after Neurosurgery
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
- 2Division of Infectious Diseases, Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sangho@amc.seoul.kr
- KMID: 2170430
- DOI: http://doi.org/10.3947/ic.2013.45.3.308
Abstract
- BACKGROUND
Postoperative bacterial meningitis (PBM) is a serious potential complication after neurosurgery. Early diagnosis and introduction of antimicrobial therapy are necessary to reduce the rate of fatal outcomes from PBM. However, PBM is not easily differentiated from postoperative aseptic meningitis (PAM), which usually has favorable clinical outcomes. Serum procalcitonin (S-PCT) has been found to be a useful marker for distinguishing community-acquired bacterial from viral meningitis. We investigated the predictive performance of S-PCT for PBM in patients who underwent neurosurgery.
MATERIALS AND METHODS
Between September 2009 and August 2010, we prospectively collected data from patients who underwent neurosurgery and had cerebrospinal fluid (CSF) pleocytosis within 14 days of surgery. Based on the CSF culture results, patients were categorized as either PBM or PAM cases. We compared the laboratory test results including S-PCT levels between PBM and PAM cases, and investigated the predictive performance of S-PCT for PBM.
RESULTS
During the study period, PBM and PAM occurred in 14 and 64 patients, respectively. There was no significant difference in CSF profiles between PBM and PAM cases. S-PCT level > or = 0.15 ng/mL (50.0% vs. 20.0%, P = 0.07) and C-reactive protein (CRP) level > or = 2.5 mg/dL (75.0% vs. 46.5%, P = 0.16) tended to be more frequent in PBM than in PAM cases. A blood white blood cell (B-WBC) count > or = 9,500/mm3 was more frequently found in PBM cases (85.7% vs. 50.8%, P = 0.02) than in PAM cases. For the diagnosis of PBM, an S-PCT level > or = 0.15 ng/mL had a specificity of 80.0%. The combined criteria of a CRP level > or = 2.5 mg/dL, B-WBC count > or = 9,500/mm3, and an S-PCT level > or = 0.15 ng/mL had the highest specificity (92.6%) of all the criteria. An S-PCT level > or =0.15 ng/mL had low sensitivity (50.0%), and the combined criteria of CRP level > or = 2.5 mg/dL, B-WBC count > or = 9,500/mm3, and S-PCT level > or = 0.15 ng/mL had an improved sensitivity of 85.7%. However, the sensitivity did not significantly differ from that of a B-WBC count > or = 9,500/mm3 (85.7%).
CONCLUSIONS
S-PCT showed limited performance for the diagnosis of postoperative meningitis. However, it could be a useful adjunct for the improvement of diagnostic sensitivity when used in combination with other inflammatory markers.
Keyword
MeSH Terms
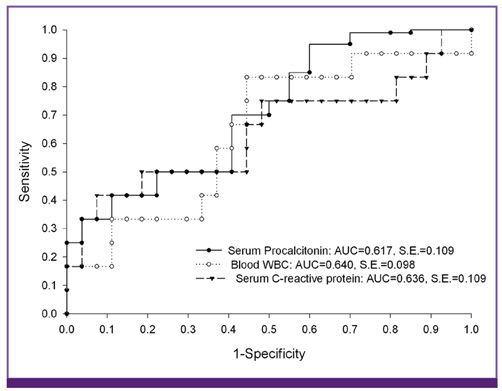
Figure
Cited by 1 articles
-
Procalcitonin in Diagnosis of Post-Operative Bacterial Meningitis: A Promising but Limited Role
Hee Jung Choi
Infect Chemother. 2013;45(3):346-348. doi: 10.3947/ic.2013.45.3.346.
Reference
-
1. Korinek AM. The French Study Group of Neurosurgical Infections, the SEHP, and the C-CLIN Paris-Nord. Service Epidémiologie Hygiène et Prévention. Risk factors for neurosurgical site infections after craniotomy: a prospective multicenter study of 2944 patients. Neurosurgery. 1997; 41:1073–1079. discussion 1079-81.
Article2. Blomstedt GC. Postoperative aseptic meningitis. Acta Neurochir (Wien). 1987; 89:112–116.
Article3. Carmel PW, Greif LK. The aseptic meningitis syndrome: a complication of posterior fossa surgery. Pediatr Neurosurg. 1993; 19:276–280.
Article4. Buckwold FJ, Hand R, Hansebout RR. Hospital-acquired bacterial meningitis in neurosurgical patients. J Neurosurg. 1977; 46:494–500.
Article5. Federico G, Tumbarello M, Spanu T, Rosell R, Iacoangeli M, Scerrati M, Tacconelli E. Risk factors and prognostic indicators of bacterial meningitis in a cohort of 3580 postneurosurgical patients. Scand J Infect Dis. 2001; 33:533–537.
Article6. Zarrouk V, Vassor I, Bert F, Bouccara D, Kalamarides M, Bendersky N, Redondo A, Sterkers O, Fantin B. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007; 44:1555–1559.
Article7. Ross D, Rosegay H, Pons V. Differentiation of aseptic and bacterial meningitis in postoperative neurosurgical patients. J Neurosurg. 1988; 69:669–674.
Article8. Leib SL, Boscacci R, Gratzl O, Zimmerli W. Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis. 1999; 29:69–74.
Article9. Forgacs P, Geyer CA, Freidberg SR. Characterization of chemical meningitis after neurological surgery. Clin Infect Dis. 2001; 32:179–185.
Article10. Tavares WM, Machado AG, Matushita H, Plese JP. CSF markers for diagnosis of bacterial meningitis in neurosurgical postoperative patients. Arq Neuropsiquiatr. 2006; 64:592–595.
Article11. Dubos F, Moulin F, Gajdos V, De Suremain N, Biscardi S, Lebon P, Raymond J, Breart G, Gendrel D, Chalumeau M. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr. 2006; 149:72–76.
Article12. Dubos F, Korczowski B, Aygun DA, Martinot A, Prat C, Galetto-Lacour A, Casado-Flores J, Taskin E, Leclerc F, Rodrigo C, Gervaix A, Leroy S, Gendrel D, Bréart G, Chalumeau M. Serum procalcitonin level and other biologic markers to distinguish between bacterial and aseptic meningitis in children: a European multicenter case cohort study. Arch Pediatr Adolesc Med. 2008; 162:1157–1163.
Article13. Viallon A, Desseigne N, Marjollet O, Birynczyk A, Belin M, Guyomarch S, Borg J, Pozetto B, Bertrand JC, Zeni F. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Crit Care. 2011; 15:R136.
Article14. Alkholi UM, Al-Monem NA, Abd El-Azim AA, Sultan MH. Serum procalcitonin in viral and bacterial meningitis. J Glob Infect Dis. 2011; 3:14–18.
Article15. Choi SH, Kim YS, Bae IG, Chung JW, Lee MS, Kang JM, Ryu J, Woo JH. The possible role of cerebrospinal fluid adenosine deaminase activity in the diagnosis of tuberculous meningitis in adults. Clin Neurol Neurosurg. 2002; 104:10–15.
Article16. Sun Q, Sha W, Xiao HP, Tian Q, Zhu H. Evaluation of cerebrospinal fluid adenosine deaminase activity for the differential diagnosis of tuberculosis and nontuberculous meningitis. Am J Med Sci. 2012; 344:116–121.
Article17. Tunkel AR. Approach to the patient with central nervous system infection. In : Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 7th ed. Philadelphia: Churchil Livingstone;2009.18. Kaufman BA, Tunkel AR, Pryor JC, Dacey RG Jr. Meningitis in the neurosurgical patient. Infect Dis Clin North Am. 1990; 4:677–701.
Article19. Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993; 341:515–518.
Article20. Gendrel D, Raymond J, Assicot M, Moulin F, Iniguez JL, Lebon P, Bohuon C. Measurement of procalcitonin levels in children with bacterial or viral meningitis. Clin Infect Dis. 1997; 24:1240–1242.
Article21. van Rossum AM, Wulkan RW, Oudesluys-Murphy AM. Procalcitonin as an early marker of infection in neonates and children. Lancet Infect Dis. 2004; 4:620–630.
Article22. Simon L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis. 2004; 39:206–217.
Article23. Hoffmann O, Reuter U, Masuhr F, Holtkamp M, Kassim N, Weber JR. Low sensitivity of serum procalcitonin in bacterial meningitis in adults. Scand J Infect Dis. 2001; 33:215–218.
Article24. Infection in Neurosurgery Working Party of the British Society for Antimicrobial Chemotherapy. The management of neurosurgical patients with postoperative bacterial or aseptic meningitis or external ventricular drain-associated ventriculitis. Br J Neurosurg. 2000; 14:7–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Procalcitonin as a Diagnostic and Prognostic Factor for Tuberculosis Meningitis
- Serum Procalcitonin and C-reactive Protein Level as an Early Diagnostic Marker of Bacterial Meningitis in the Emergency Department
- Applying the Bacterial Meningitis Score in Neonates Diagnosed Meningitis: A Single Center Experience
- Sweet's Syndrome Associated with Bacterial Meningitis
- Predictive Value of C-Reactive Protein in the Differential Diagnosis of Acute Meningitis in Adults