Anat Cell Biol.
2010 Sep;43(3):211-217. 10.5115/acb.2010.43.3.211.
Differential regulation of Purkinje cell dendritic spines in rolling mouse Nagoya (tg(rol)/tg(rol)), P/Q type calcium channel (alpha1(A)/Ca(v)2.1) mutant
- Affiliations
-
- 1Laboratory of Animal Management, School of Agricultural Science, Nagoya University, Nagoya, Japan.
- 2Department of Anatomy, College of Medicine and Human Genetics, Korea University, Seoul, Korea. irhyu@korea.ac.kr
- 3Section of Brain Structure, Center for Brain Experiment, National Institute for Physiological Sciences, Okazaki, Japan.
- 4Department of Information Physiology, National Institute for Physiological Sciences, Okazaki, Japan.
- 5Genetic Resource Center, Korea Research Institute of Bioscience and Biotechnology, KRIBB, Daejon, Korea.
- KMID: 2168876
- DOI: http://doi.org/10.5115/acb.2010.43.3.211
Abstract
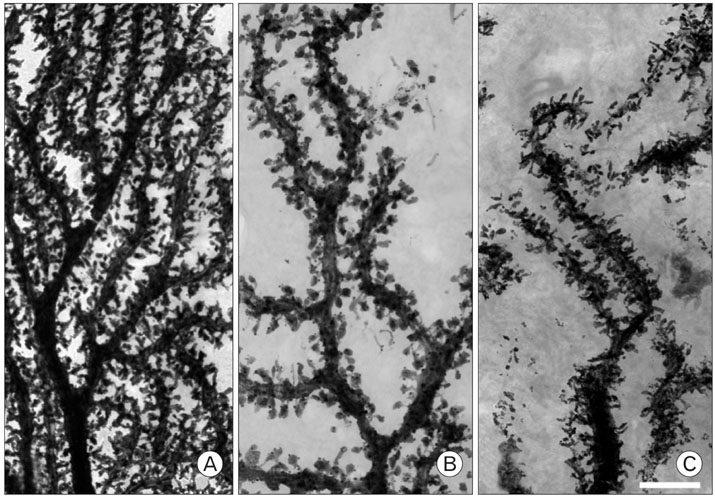
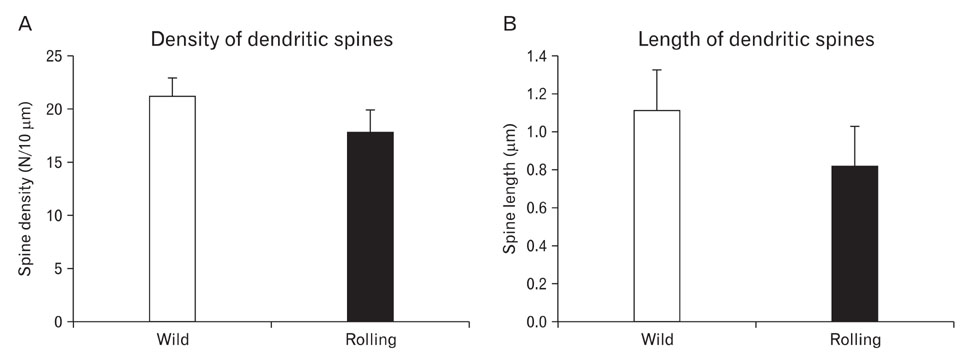
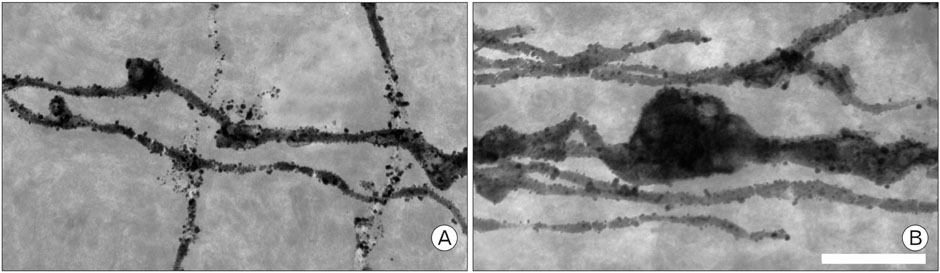
- Voltage dependent calcium channels (VDCC) participate in regulation of neuronal Ca2+. The Rolling mouse Nagoya (Cacna1a(tg-rol) ) is a spontaneous P/Q type VDCC mutant, which has been suggested as an animal model for some human neurological diseases such as autosomal dominant cerebellar ataxia (SCA6), familial hemiplegic migraine and episodic ataxia type-2. Morphology of Purkinje cell (PC) dendritic spine is suggested to be regulated by signal molecules such as Ca2+ and by interactions with afferent inputs. The amplitude of excitatory postsynaptic current was decreased in parallel fiber (PF) to PC synapses, whereas apparently increased in climbing fiber (CF) to PC synapses in rolling mice Nagoya. We have studied synaptic morphology changes in cerebella of this mutant strain. We previously found altered synapses between PF varicosity and PC dendritic spines. To study dendritic spine plasticity of PC in the condition of insufficient P/Q type VDCC function, we used high voltage electron microscopy (HVEM). We measured the density and length of PC dendritic spines at tertiary braches. We observed statistically a significant decrease in spine density as well as shorter spine length in rolling mice compared to wild type mice at tertiary dendritic braches. In proximal PC dendrites, however, there were more numerous dendritic spines in rolling mice Nagoya. The differential regulation of rolling PC spines at tertiary and proximal dendrites in rolling mice Nagoya suggests that two major excitatory afferent systems may be regulated reciprocally in the cerebellum of rolling mouse Nagoya.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Histological features of the Purkinje neurons of the Albino rat (
Rattus norvegicus ) following letrozole administration
Chaudhry Talha Hannan, Munguti Kilonzo Jeremiah, Pamela Mandela Idenya
Anat Cell Biol. 2025;58(1):76-85. doi: 10.5115/acb.24.088.
Reference
-
1. Austin MC, Schultzberg M, Abbott LC, et al. Expression of tyrosine hydroxylase in cerebellar Purkinje neurons of the mutant tottering and leaner mouse. Brain Res Mol Brain Res. 1992. 15:227–240.2. Cicale M, Ambesi-Impiombato A, Cimini V, et al. Decreased gene expression of calretinin and ryanodine receptor type 1 in tottering mice. Brain Res Bull. 2002. 59:53–58.3. Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000. 25:533–535.4. Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995. 268:239–247.5. Hama K, Arii T, Kosaka T. Three-dimensional morphometrical study of dendritic spines of the granule cell in the rat dentate gyrus with HVEM stereo images. J Electron Microsc Tech. 1989. 12:80–87.6. Hama K, Kosaka T. Zimmerman HM, editor. Purkinje cell and related neurons and glial cells under high voltage electron microscopy. Progress in neuropathology. 1979. New York: Raven Press;61–77.7. Harris KM, Stevens JK. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1988. 8:4455–4469.8. Huang CM, Brown N, Huang RH. Age-related changes in the cerebellum: parallel fibers. Brain Res. 1999. 840:148–152.9. Isaacs KR, Abbott LC. Cerebellar volume decreases in the tottering mouse are specific to the molecular layer. Brain Res Bull. 1995. 36:309–314.10. Jun K, Piedras-Rentería ES, Smith SM, et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA. 1999. 96:15245–15250.11. Lee KJ, Kim H, Kim TS, Park SH, Rhyu IJ. Morphological analysis of spine shapes of Purkinje cell dendrites in the rat cerebellum using high-voltage electron microscopy. Neurosci Lett. 2004. 359:21–24.12. Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988. 242:1654–1664.13. Matsushita K, Wakamori M, Rhyu IJ, et al. Bidirectional alterations in cerebellar synaptic transmission of tottering and rolling Ca2+ channel mutant mice. J Neurosci. 2002. 22:4388–4398.14. Mori Y, Wakamori M, Oda S, et al. Reduced voltage sensitivity of activation of P/Q-type Ca2+ channels is associated with the ataxic mouse mutation rolling Nagoya (tg(rol)). J Neurosci. 2000. 20:5654–5662.15. Oda S. The observation of rolling mouse Nagoya (rol), a new neurological mutant, and its maintenance (author's transl). Jikken Dobutsu. 1973. 22:281–288.16. Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996. 87:543–552.17. Palay SL, Chan-Palay V. Cerebellar cortex - cytology and organization. 1974. New York, Berlin: Springer-Verlag.18. Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002. 25:31–50.19. Rhyu IJ, Abbott LC, Walker DB, Sotelo C. An ultrastructural study of granule cell/Purkinje cell synapses in tottering (tg/tg), leaner (tg(la)/tg(la)) and compound heterozygous tottering/leaner (tg/tg(la)) mice. Neuroscience. 1999a. 90:717–728.20. Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999b. 270:149–152.21. Rhyu IJ, Nahm SS, Hwang SJ, et al. Altered neuronal nitric oxide synthase expression in the cerebellum of calcium channel mutant mice. Brain Res. 2003. 977:129–140.22. Rhyu IJ, Oda S, Uhm CS, Kim H, Suh YS, Abbott LC. Morphologic investigation of rolling mouse Nagoya (tg(rol)/tg(rol)) cerebellar Purkinje cells: an ataxic mutant, revisited. Neurosci Lett. 1999c. 266:49–52.23. Segal I, Korkotian I, Murphy DD. Dendritic spine formation and pruning: common cellular mechanisms? Trends Neurosci. 2000. 23:53–57.24. Strata P, Morando L, Bravin M, Rossi F. Dendritic spine density in Purkinje cells. Trends Neurosci. 2000. 23:198.25. Suh YS, Oda S, Kang YH, Kim H, Rhyu IJ. Apoptotic cell death of cerebellar granule cells in rolling mouse Nagoya. Neurosci Lett. 2002. 325:1–4.26. Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990. 6:715–760.27. Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR. Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur J Neurosci. 2000. 12:945–954.28. Westenbroek RE, Sakurai T, Elliott EM, et al. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995. 15:6403–6418.29. Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994. 264:107–111.30. Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997. 15:62–69.31. Zwingman TA, Neumann PE, Noebels JL, Herrup K. Rocker is a new variant of the voltage-dependent calcium channel gene Cacna1a. J Neurosci. 2001. 21:1169–1178.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Plasticity of synapses between Purkinje cell dendritic spines and parallel fiber varicosity in tottering/leaner mice cerebellum
- Voltage-dependent Calcium Channel (VDCC) alpha(1A) Subunit Expression in the Ataxic Mutant, Pogo Mice Cerebellum
- Absence Expression of Heat Shock Protein 25 in the Cerebellum of Ataxic Mutant Pogo Mouse
- Carnosine and Retinol Synergistically Inhibit UVB-Induced PGE2 Synthesis in Human Keratinocytes through the Up-Regulation of Hyaluronan Synthase 2
- Initiation Site of Ca2+ Entry Evoked by Endoplasmic Reticulum Ca2+ Depletion in Mouse Parotid and Pancreatic Acinar Cells