Anat Cell Biol.
2011 Mar;44(1):41-49. 10.5115/acb.2011.44.1.41.
Transient lysosomal activation is essential for p75 nerve growth factor receptor expression in myelinated Schwann cells during Wallerian degeneration
- Affiliations
-
- 1Department of Physiology, Mitochondria Hub Research Center, College of Medicine, Dong-A University, Busan, Korea. phwantae@dau.ac.kr
- 2Department of Neurology, Mitochondria Hub Research Center, College of Medicine, Dong-A University, Busan, Korea.
- KMID: 2168867
- DOI: http://doi.org/10.5115/acb.2011.44.1.41
Abstract
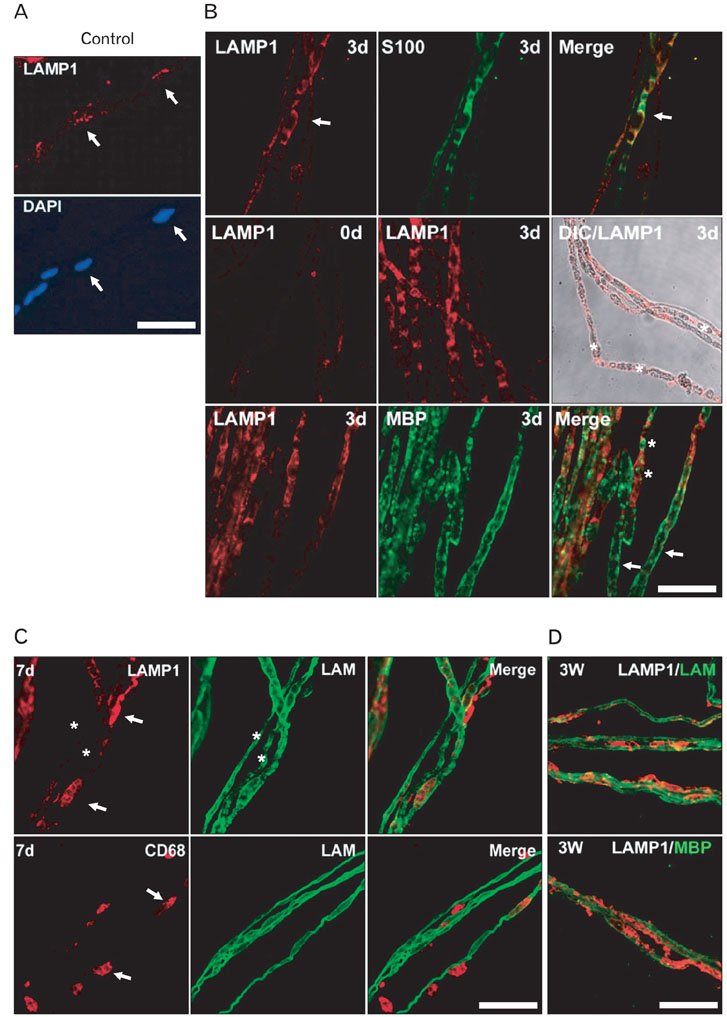
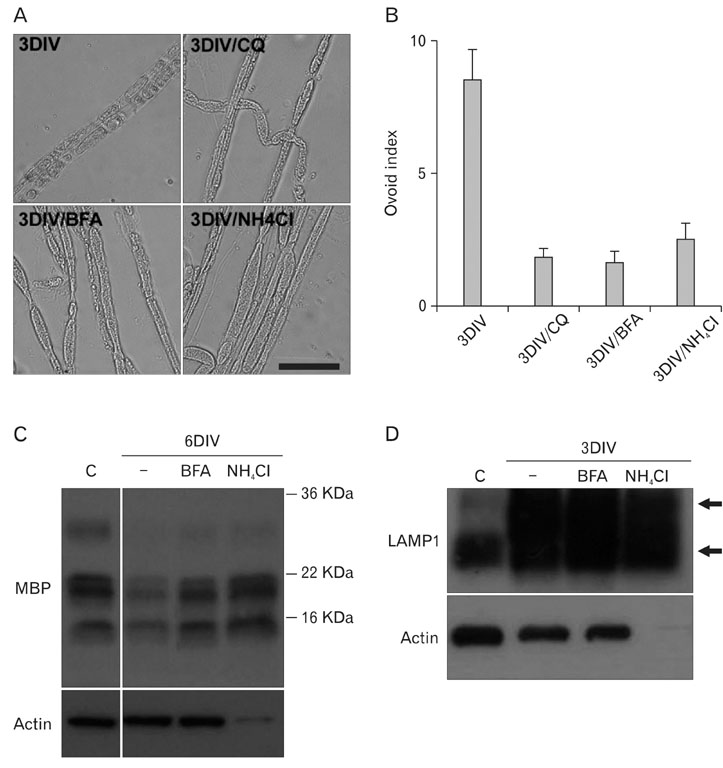
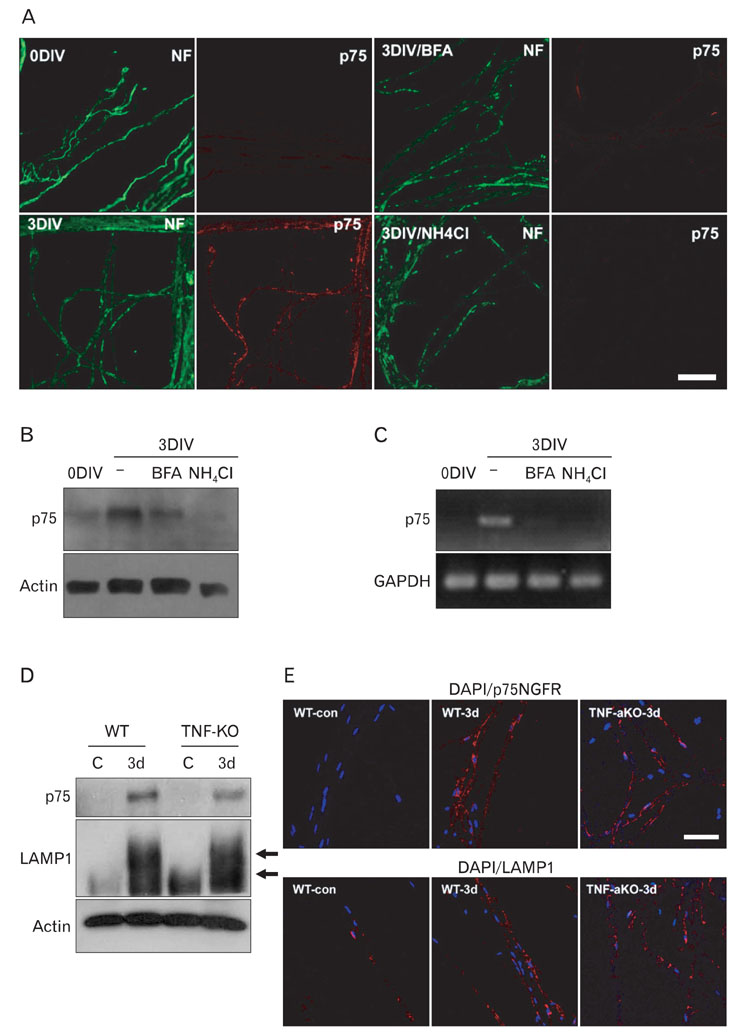
- Myelinated Schwann cells in the peripheral nervous system express the p75 nerve growth factor receptor (p75NGFR) as a consequence of Schwann cell dedifferentiation during Wallerian degeneration. p75NGFR has been implicated in the remyelination of regenerating nerves. Although many studies have shown various mechanisms underlying Schwann cell dedifferentiation, the molecular mechanism contributing to the re-expression of p75NGFR in differentiated Schwann cells is largely unknown. In the present study, we found that lysosomes were transiently activated in Schwann cells after nerve injury and that the inhibition of lysosomal activation by chloroquine or lysosomal acidification inhibitors prevented p75NGFR expression at the mRNA transcriptional level in an ex vivo Wallerian degeneration model. Lysosomal acidification inhibitors suppressed demyelination, but not axonal degeneration, thereby suggesting that demyelination mediated by lysosomes may be an important signal for inducing p75NGFR expression. Tumor necrosis factor-alpha (TNF-alpha) has been suggested to be involved in regulating p75NGFR expression in Schwann cells. In this study, we found that removing TNF-alpha in vivo did not significantly suppress the induction of both lysosomes and p75NGFR. Thus, these findings suggest that lysosomal activation is tightly correlated with the induction of p75NGFR in demyelinating Schwann cells during Wallerian degeneration.
Keyword
MeSH Terms
Figure
Reference
-
1. Martini R, Fischer S, López-Vales R, David S. Interactions between Schwann cells and macrophages in injury and inherited demyelinating disease. Glia. 2008. 56:1566–1577.2. Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994. 14(5 Pt 2):3231–3245.3. Hallpike JF, Adams CW. Proteolysis and myelin breakdown: a review of recent histochemical and biochemical studies. Histochem J. 1969. 1:559–578.4. Hallpike JF, Adams CW, Bayliss OB. Histochemistry of myelin. IX. Neutral and acid proteinases in early Wallerian degeneration. Histochem J. 1970. 2:209–218.5. Weller RO, Mellick RS. Acid phosphatase and lysosome activity in diphtheritic neuropathy and Wallerian degeneration. Br J Exp Pathol. 1966. 47:425–434.6. Lee HK, Shin YK, Jung J, Seo SY, Baek SY, Park HT. Proteasome inhibition suppresses Schwann cell dedifferentiation in vitro and in vivo. Glia. 2009. 57:1825–1834.7. Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992. 119:45–54.8. Zhang JY, Luo XG, Xian CJ, Liu ZH, Zhou XF. Endogenous BDNF is required for myelination and regeneration of injured sciatic nerve in rodents. Eur J Neurosci. 2000. 12:4171–4180.9. Hu X, He W, Diaconu C, Tang X, Kidd GJ, Macklin WB, Trapp BD, Yan R. Genetic deletion of BACE1 in mice affects remyelination of sciatic nerves. FASEB J. 2008. 22:2970–2980.10. Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009. 57:1178–1191.11. Xiao J, Kilpatrick TJ, Murray SS. The role of neurotrophins in the regulation of myelin development. Neurosignals. 2009. 17:265–276.12. Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987. 104:1623–1631.13. Taniuchi M, Clark HB, Schweitzer JB, Johnson EM Jr. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression by axonal contact, and binding properties. J Neurosci. 1988. 8:664–681.14. Song XY, Zhou FH, Zhong JH, Wu LL, Zhou XF. Knockout of p75(NTR) impairs re-myelination of injured sciatic nerve in mice. J Neurochem. 2006. 96:833–842.15. Tomita K, Kubo T, Matsuda K, Fujiwara T, Yano K, Winograd JM, Tohyama M, Hosokawa K. The neurotrophin receptor p75NTR in Schwann cells is implicated in remyelination and motor recovery after peripheral nerve injury. Glia. 2007. 55:1199–1208.16. Bolin LM, Shooter EM. Neurons regulate Schwann cell genes by diffusible molecules. J Cell Biol. 1993. 123:237–243.17. Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. J Neuroimmunol. 1993. 45:175–182.18. Schneider-Schaulies J, Kirchhoff F, Archelos J, Schachner M. Down-regulation of myelin-associated glycoprotein on Schwann cells by interferon-gamma and tumor necrosis factor-alpha affects neurite outgrowth. Neuron. 1991. 7:995–1005.19. Bonetti B, Valdo P, Stegagno C, Tanel R, Zanusso GL, Ramarli D, Fiorini E, Turazzi S, Carner M, Moretto G. Tumor necrosis factor alpha and human Schwann cells: signalling and phenotype modulation without cell death. J Neuropathol Exp Neurol. 2000. 59:74–84.20. Lisak RP, Bealmear B, Benjamins JA, Skoff AM. Interferon-gamma, tumor necrosis factor-alpha, and transforming growth factor-beta inhibit cyclic AMP-induced Schwann cell differentiation. Glia. 2001. 36:354–363.21. Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007. 102:686–698.22. Lee HK, Seo IA, Suh DJ, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009. 108:776–786.23. Michihara A, Toda K, Kubo T, Fujiwara Y, Akasaki K, Tsuji H. Disruptive effect of chloroquine on lysosomes in cultured rat hepatocytes. Biol Pharm Bull. 2005. 28:947–951.24. Boyle K, Azari MF, Cheema SS, Petratos S. TNFalpha mediates Schwann cell death by upregulating p75NTR expression without sustained activation of NFkappaB. Neurobiol Dis. 2005. 20:412–427.25. Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008. 56:1552–1565.26. Vroemen M, Weidner N. Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods. 2003. 124:135–143.27. Nakashima S, Hiraku Y, Tada-Oikawa S, Hishita T, Gabazza EC, Tamaki S, Imoto I, Adachi Y, Kawanishi S. Vacuolar H+-ATPase inhibitor induces apoptosis via lysosomal dysfunction in the human gastric cancer cell line MKN-1. J Biochem. 2003. 134:359–364.28. Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol Chem. 2002. 383:1827–1831.29. Stoll G, Li CY, Trapp BD, Griffin JW. Expression of NGF-receptors during immune-mediated and lysolecithin-induced demyelination of the peripheral nervous system. J Neurocytol. 1993. 22:1022–1029.30. Toews AD, Griffiths IR, Kyriakides E, Goodrum JF, Eckermann CE, Morell P, Thomson CE. Primary demyelination induced by exposure to tellurium alters Schwann cell gene expression: a model for intracellular targeting of NGF receptor. J Neurosci. 1992. 12:3676–3687.31. Hanemann CO, Gabreëls-Fasten AA, Müller HW, Stoll G. Low affinity NGF receptor expression in CMT1A nerve biopsies of different disease stages. Brain. 1996. 119(Pt 5):1461–1469.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Double-stranded RNA Induces Inflammatory Gene Expression in Schwann Cells: Implication in the Wallerian Degeneration
- Chiral 1,4-oxazepan-3-one Targeting Schwann Cells Exhibits Morphometrically Inhibitory Effects on Wallerian Degeneration
- Ultrastructural Findings of Cultured Ciliary Nerve
- Change in the Expression of p75 Neurotrophin Receptor and TRPV1 in the Spinal Cord and Dorsal Root Ganglion after an Injury to the Spinal Nerves in Rats
- The Morphological Studies on the Effects of Nerve Growth Factor on the Schwann Cell in the Diabetic Neuropathy in the Rats