J Bacteriol Virol.
2015 Mar;45(1):54-61. 10.4167/jbv.2015.45.1.54.
Development and Verification of Nested PCR Assay for Detection of Tobacco rattle virus in Plant Quarantine
- Affiliations
-
- 1Environmental Infrastructure Research Department, National Institute of Environmental Research, Incheon, Korea.
- 2Incheon International Airport Regional Office, Animal and Plant Quarantine Agency, Incheon, Korea.
- 3School of Applied Biosciences, Kyungpook National University, Daegu, Korea. suheon@knu.ac.kr
- 4Department of Microbiology, College of Natural Sciences, Dankook University, Cheonan, Korea. ahnty@dankook.ac.kr
- KMID: 2168705
- DOI: http://doi.org/10.4167/jbv.2015.45.1.54
Abstract
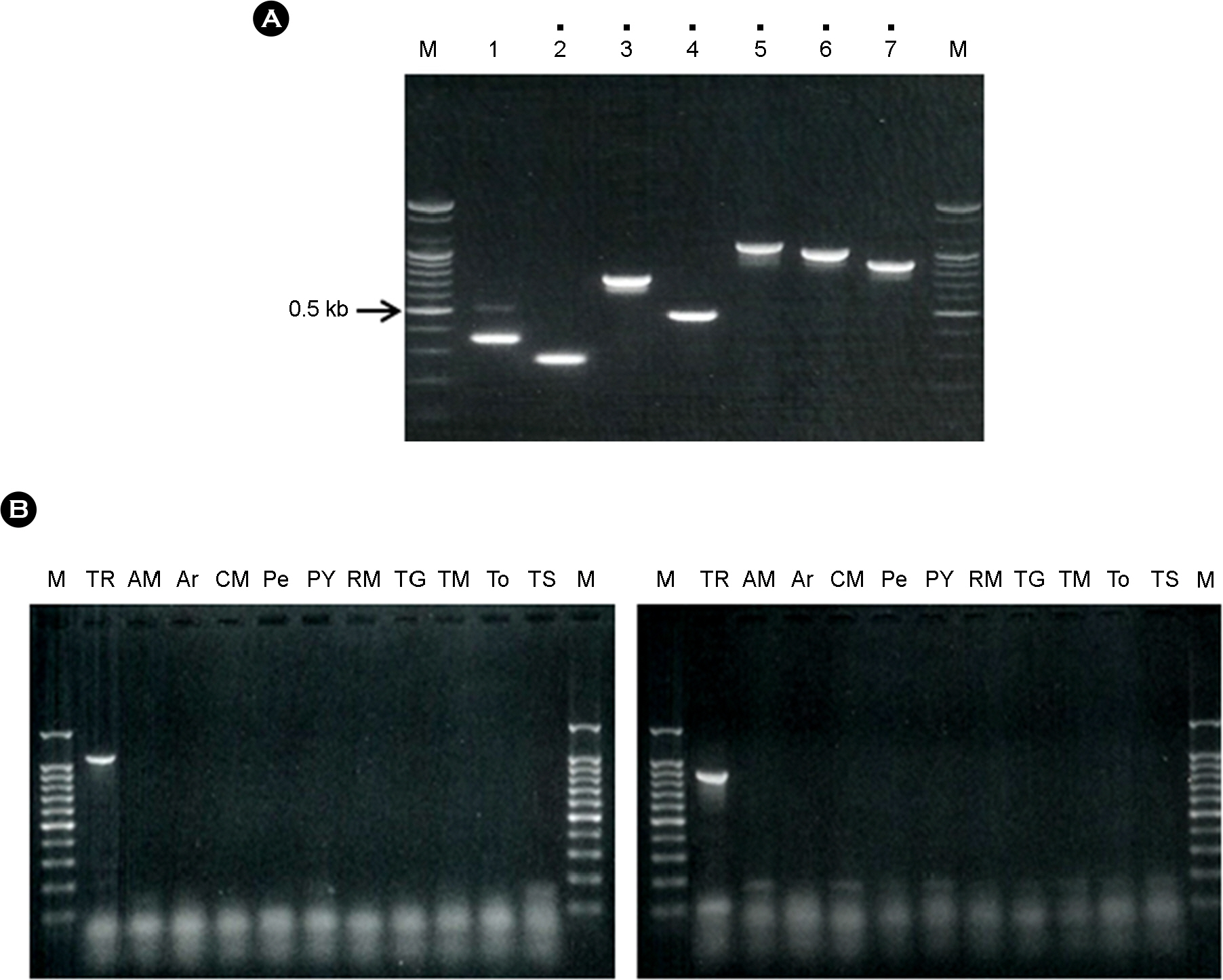
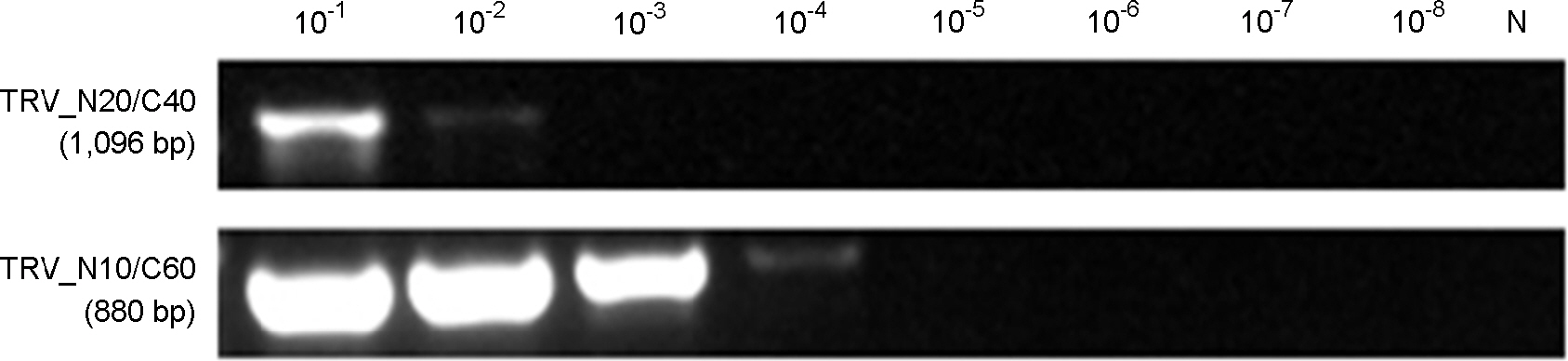
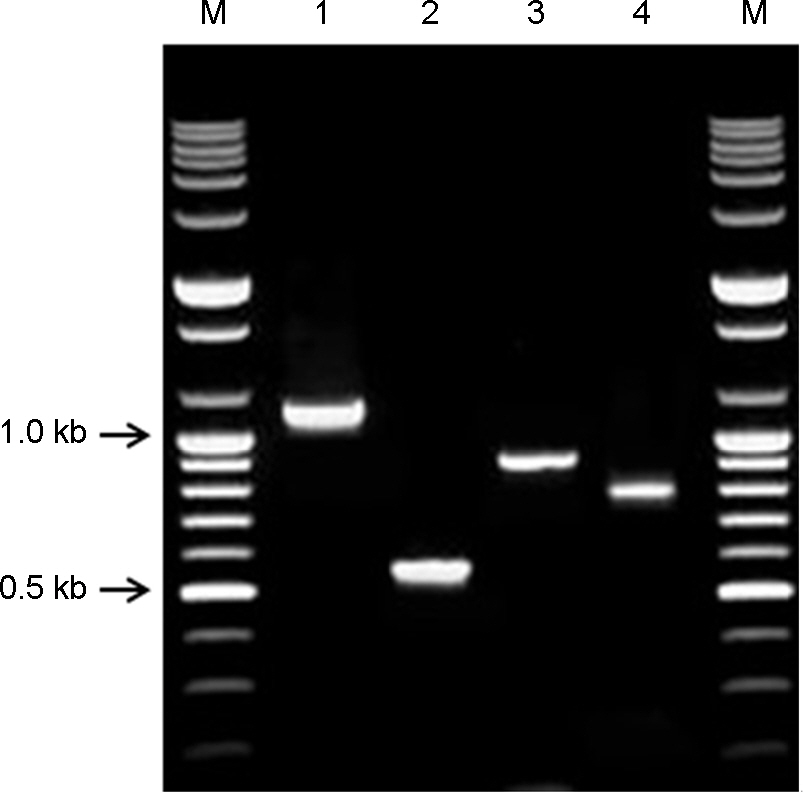
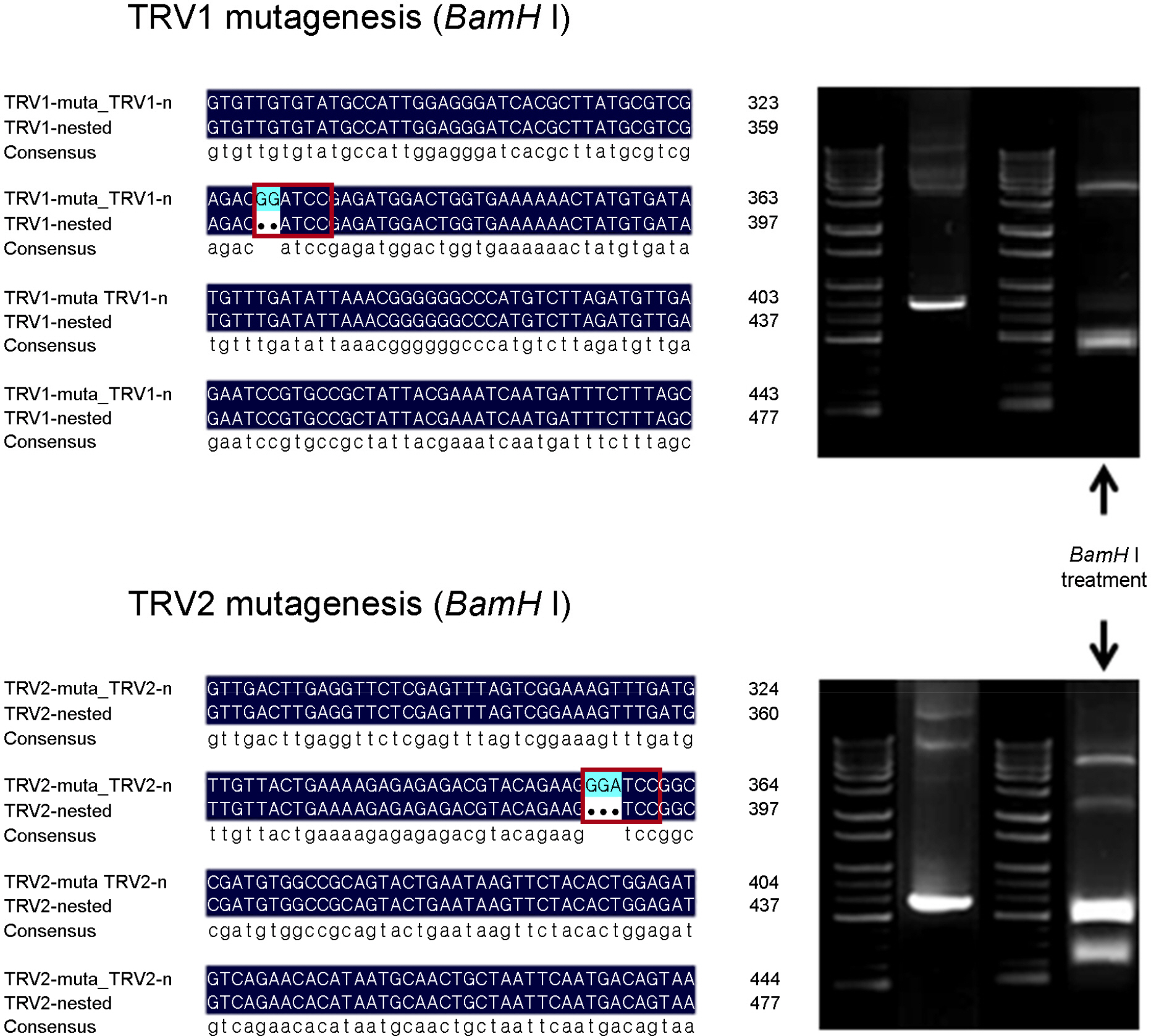
- Tobacco rattle virus (TRV) is a plant pathogen belonging to the Group IV positive-sense single-stranded RNA viruses. TRV causes disease in various plants (e.g., potato, tomato and tobacco), for which it was classified as a controlled quarantine virus in Korea. This study aimed to develop specific primer sets for the rapid detection of TRV. Two RT-PCR primer sets were developed for specific detection of TRV. Furthermore, nested primer sets were also developed, which is required for high sensitivity detection in plant quarantine. The RT-PCR and nested PCR products had the following sizes: set 5 (1,096-->540 bp), and set 7 (878-->756 bp), respectively. In addition, a modified positive-control plasmid was also developed for use as a positive control in TRV quarantine. The diagnostic system for TRV detection was verified using samples from Korean quarantine sites for the last five years (2009-2014). A total of 83 cases were detected among various import crops. This system for detection of TRV will continuously contribute to plant quarantine in the future.
Keyword
MeSH Terms
Figure
Reference
-
1). Lee S, Kang EH, Heo NY, Kim SM, Kim YJ, Shin YG. Detection of Carnation necrotic fleck virus and Carnation ringspot virus using RT-PCR. Res Plant Dis. 2013; 19:36–44.2). Lee S, Kang EH, Chu YM, Shin YG, Ahn TY. Development of PCR diagnosis system for plant quarantine seed-borne Wheat streak mosaic virus. Korean J Microbiol. 2013; 49:112–7.3). Lee S, Shin YG. Development and practical use of RT-PCR for seed-transmitted Prune dwarf virus in quarantine. Plant Pathol J. 2014; 30:178–82.4). Nicolaisen M, Bösze Z, Nielsen SL. Detection of Tobacco rattle virus in potato tubers using a simple RT-PCR procedure. Potato Research. 1999; 42:173–9.5). Wei T, Lu G, Clover GRG. A multiplex RT-PCR for the detection of Potato yellow vein virus, Tobacco rattle virus and Tomato infectious chlorosis virus in potato with a plant internal amplification control. Plant Pathology. 2009; 58:203–9.6). Pérez EE, Weingartner DP, Hiebert E, McSorley R. Tobacco rattle virus detection in potato tubers from northeast Florida by PCR and tissue blotting. Amer J of Potato Res. 2012; 77:363–8.7). Riga E, Larsen R, Eastwell K, Guerra N, Guerra L, Crosslin JM. Rapid detection of Tobacco rattle tobravirus in Viruliferous paratrichodorus allius from greenhouse and field specimens. J Nematol. 2009; 41:60–3.8). Kenyon DM, Handy CL, Thomas JE. Detection of Tobacco rattle virus in soil using real-time PCR. Aspects of Applied Biology. 2005; 76:77–9.9). Yang JG, Wang FL, Chen DX, Shen LL, Qian YM, Liang ZY, et al. Development of a one-step immunocapture real-time RT-PCR assay for detection of Tobacco mosaic virus in soil. Sensors. 2012; 12:16685–94.10). Xu H, Nie J. Molecular detection and identification of potato isolates of Tobacco rattle virus. Canadian J Plant Pathol. 2006; 28:271–9.11). Holeva R, Phillips MS, Neilson R, Brown DJF, Young V, Boutsika K, et al. Real-time PCR detection and quantification of vector trichodorid nematodes and Tobacco rattle virus. Mol Cell Probes. 2006; 20:203–11.12). Mumford RA, Walsh K, Barker I, Boonham N. Detection of Potato mop top virus and Tobacco rattle virus using a multiplex real-time fluorescent reverse-transcription polymerase chain reaction assay. Phytopathology. 2000; 90:448–53.13). Shin YG, Rho JY. Development of a PCR diagnostic system for Iris yellow spot tospovirus in quarantine. Plant Pathol J. 2014; 30:440–4.14). Animal, Plant and Fisheries Quarantine and Inspection Agency. List of plant quarantine viruses in Korea newly revised in 2013. Res Plant Dis. 2013; 19:67–75.15). Shin YG. An advanced quarantine system for the inspection of seed-borne viruses in Korea. 2009.16). Lee S, Kang EH, Shin YG, Lee SH. Development of RT-PCR and nested PCR for detecting four quarantine plant viruses belonging to Nepovirus. Res Plant Dis. 2013; 19:220–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and evaluation of an immunochromatographic assay using a gp51 monoclonal antibody for the detection of antibodies against the bovine leukemia virus
- Monitoring of Five Bovine Arboviral Diseases Transmitted by Arthropod Vectors in Korea
- Detection of Varicella-Zoster Virus (VZV) by Nested Polymerase Chain Reaction
- Detection of canine distemper virus (CDV) through one step RT-PCR combined with nested PCR
- Establishment of a Multiplex RT-PCR for the Sensitive and Differential Detection of Japanese Encephalitis Virus Genotype 1 and 3