Immune Netw.
2015 Aug;15(4):206-211. 10.4110/in.2015.15.4.206.
Involvement of Protein Kinase C-delta in Vascular Permeability in Acute Lung Injury
- Affiliations
-
- 1Department of Internal Medicine, Ulsan University Hospital, School of Medicine, University of Ulsan, Ulsan 44033, Korea.
- 2Department of Thoracic Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 44033, Korea.
- 3Department of Anesthesiology and Pain Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 44033, Korea.
- 4School of Biological Sciences, University of Ulsan, Ulsan 44610, Korea. bkwon@ulsan.ac.kr
- 5Biomedical Research Center, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 44033, Korea. hrcho@uuh.ulsan.kr
- 6Department of Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan 44033, Korea.
- KMID: 2168048
- DOI: http://doi.org/10.4110/in.2015.15.4.206
Abstract
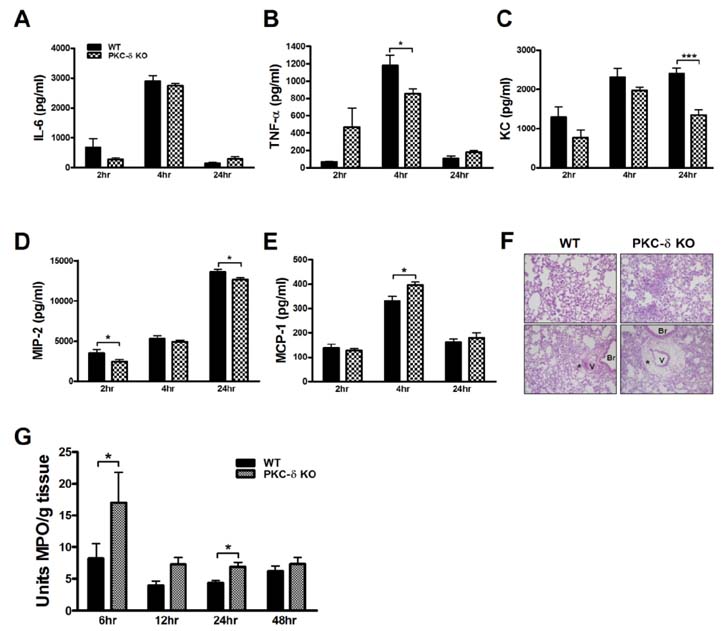
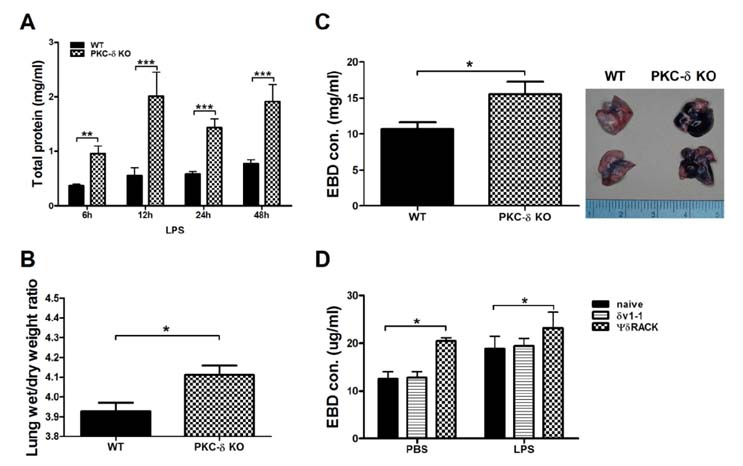
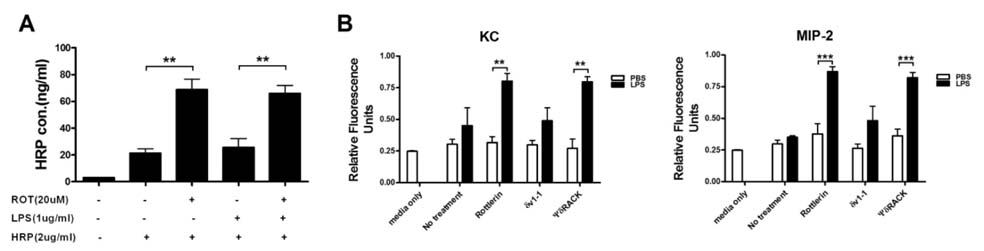
- Pulmonary edema is a major cause of mortality due to acute lung injury (ALI). The involvement of protein kinase C-delta (PKC-delta) in ALI has been a controversial topic. Here we investigated PKC-delta function in ALI using PKC-delta knockout (KO) mice and PKC inhibitors. Our results indicated that although the ability to produce proinflammatory mediators in response to LPS injury in PKC-delta KO mice was similar to that of control mice, they showed enhanced recruitment of neutrophils to the lung and more severe pulmonary edema. PKC-delta inhibition promoted barrier dysfunction in an endothelial cell layer in vitro, and administration of a PKC-delta-specific inhibitor significantly increased steady state vascular permeability. A neutrophil transmigration assay indicated that the PKC-delta inhibition increased neutrophil transmigration through an endothelial monolayer. This suggests that PKC-delta inhibition induces structural changes in endothelial cells, allowing extravasation of proteins and neutrophils.
MeSH Terms
Figure
Reference
-
1. Han S, Mallampalli RK. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015; 194:855–860.
Article2. Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003; 284:L435–L451.
Article3. Klinger JR, Murray JD, Casserly B, Alvarez DF, King JA, An SS, Choudhary G, Owusu-Sarfo AN, Warburton R, Harrington EO. Rottlerin causes pulmonary edema in vivo: a possible role for PKCδ. J Appl Physiol. 2007; 103:2084–2094.
Article4. Gaudreault N, Perrin RM, Guo M, Clanton CP, Wu MH, Yuan SY. Counter regulatory effects of PKCβII and PKCδ on coronary endothelial permeability. Arterioscler Thromb Vasc Biol. 2008; 28:1527–1533.
Article5. Chichger H, Grinnell KL, Casserly B, Chung CS, Braza J, Lomas-Neira J, Ayala A, Rounds S, Klinger JR, Harrington EO. Genetic disruption of protein kinase Cδ reduces endotoxin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2012; 303:L880–L888.
Article6. Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002; 416:865–869.
Article7. Lomas-Neira J, Chung CS, Grutkoski PS, Dunican A, Simms HH, Cioffi WG, Ayala A. Divergent roles of murine neutrophil chemokines in hemorrhage induced priming for acute lung injury. Cytokine. 2005; 31:169–179.
Article8. Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, Bernacki KD, Huber-Lang M, McGuire S, Hoesel LM, Riedemann NC, Beck-Schimmer B, Zetoune FS, Ward PA. Relationship of acute lung inflammatory injury to Fas/FasL system. Am J Pathol. 2005; 166:685–694.
Article9. Patterson CE, Rhoades RA, Garcia JG. Evans blue dye as a marker of albumin clearance in cultured endothelial monolayer and isolated lung. J Appl Physiol (1985). 1992; 72:865–873.
Article10. Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, Cavallaro G, Banci L, Guo Y, Bolli R, Dorn GW 2nd, Mochly-Rosen D. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci U S A. 2001; 98:11114–11119.
Article11. Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007; 2:481–485.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Protein Kinase C on Hypoxic Pulmonary Vasoconstriction in Isolated Rat Lungs
- Angiopoietin-1 variant, COMP-Ang1 attenuates hydrogen peroxide-induced acute lung injury
- Role of Protein Kinase C-delta in Atherosclerosis
- Effects of sevoflurane on tight junction protein expression and PKC-alpha translocation after pulmonary ischemia-reperfusion injury
- Morphine and remifentanil-induced cardioprotection: its experimental and clinical outcomes