Immune Netw.
2011 Oct;11(5):268-280. 10.4110/in.2011.11.5.268.
Distinct Humoral and Cellular Immunity Induced by Alternating Prime-boost Vaccination Using Plasmid DNA and Live Viral Vector Vaccines Expressing the E Protein of Dengue Virus Type 2
- Affiliations
-
- 1College of Veterinary Medicine and Bio-Safety Research Institute, Chonbuk National University, Jeonju 561-756, Korea. vetvirus@chonbuk.ac.kr
- KMID: 2167999
- DOI: http://doi.org/10.4110/in.2011.11.5.268
Abstract
- BACKGROUND
Dengue virus, which belongs to the Flavivirus genus of the Flaviviridae family, causes fatal dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) with infection risk of 2.5 billion people worldwide. However, approved vaccines are still not available. Here, we explored the immune responses induced by alternating prime-boost vaccination using DNA vaccine, adenovirus, and vaccinia virus expressing E protein of dengue virus type 2 (DenV2).
METHODS
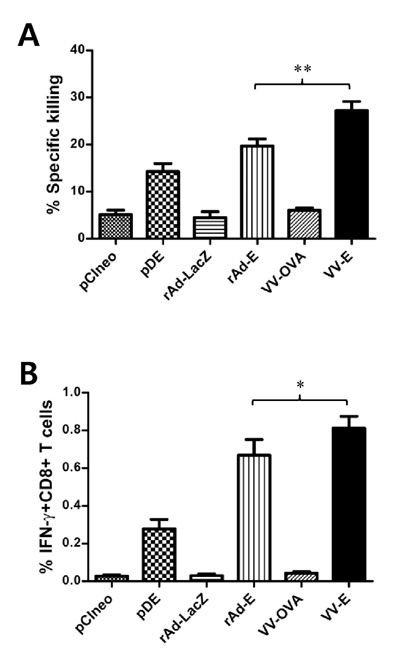
Following immunization with DNA vaccine (pDE), adenovirus (rAd-E), and/or vaccinia virus (VV-E) expressing E protein, E protein-specific IgG and its isotypes were determined by conventional ELISA. Intracellular CD154 and cytokine staining was used for enumerating CD4+ T cells specific for E protein. E protein-specific CD8+ T cell responses were evaluated by in vivo CTL killing activity and intracellular IFN-gamma staining.
RESULTS
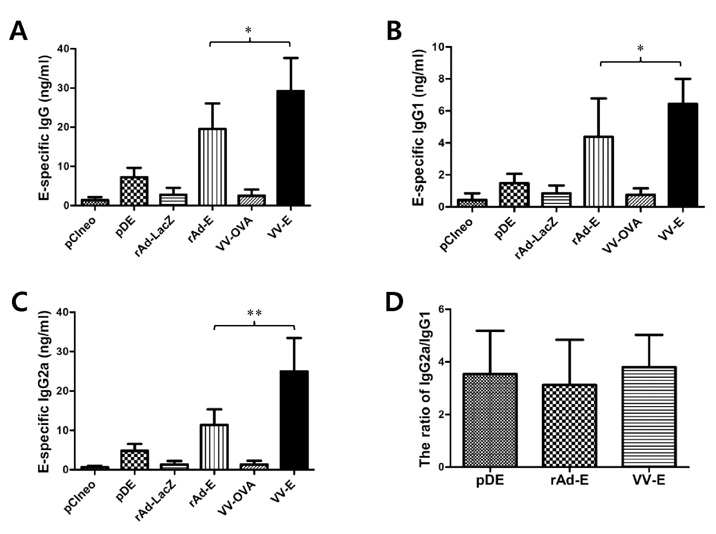
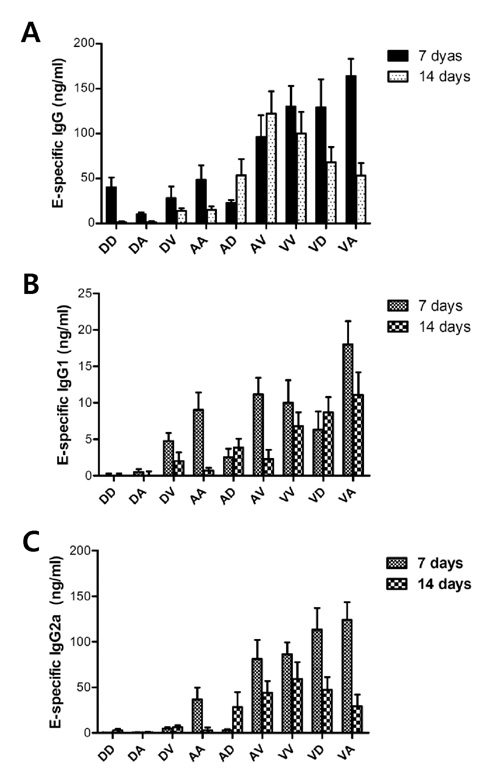
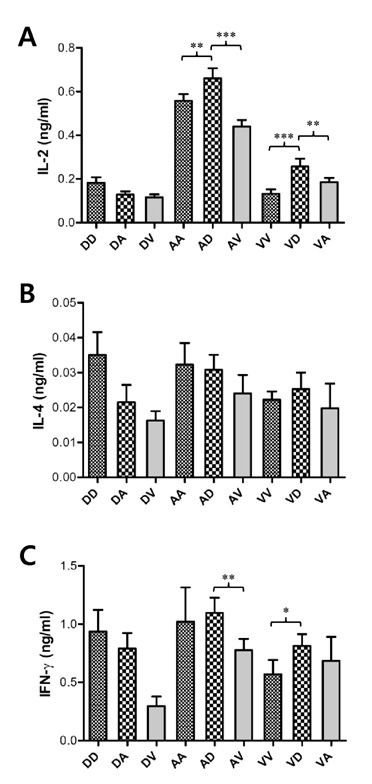
Among three constructs, VV-E induced the most potent IgG responses, Th1-type cytokine production by stimulated CD4+ T cells, and the CD8+ T cell response. Furthermore, when the three constructs were used for alternating prime-boost vaccination, the results revealed a different pattern of CD4+ and CD8+ T cell responses. i) Priming with VV-E induced higher E-specific IgG level but it was decreased rapidly. ii) Strong CD8+ T cell responses specific for E protein were induced when VV-E was used for the priming step, and such CD8+ T cell responses were significantly boosted with pDE. iii) Priming with rAd-E induced stronger CD4+ T cell responses which subsequently boosted with pDE to a greater extent than VV-E and rAd-E.
CONCLUSION
These results indicate that priming with live viral vector vaccines could induce different patterns of E protein- specific CD4+ and CD8+ T cell responses which were significantly enhanced by booster vaccination with the DNA vaccine. Therefore, our observation will provide valuable information for the establishment of optimal prime-boost vaccination against DenV.
Keyword
MeSH Terms
Figure
Reference
-
1. Swaminathan S, Khanna N. Dengue: recent advances in biology and current status of translational research. Curr Mol Med. 2009. 9:152–173.
Article2. Guha-Sapir D, Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005. 2:1.3. Halstead SB. Dengue. Lancet. 2007. 370:1644–1652.
Article4. Rothman AL. Dengue: defining protective versus pathologic immunity. J Clin Invest. 2004. 113:946–951.
Article5. Stephenson JR. Understanding dengue pathogenesis: implications for vaccine design. Bull World Health Organ. 2005. 83:308–814.6. Kanesa-thasan N, Sun W, Kim-Ahn G, Van Albert S, Putnak JR, King A, Raengsakulsrach B, Christ-Schmidt H, Gilson K, Zahradnik JM, Vaughn DW, Innis BL, Saluzzo JF, Hoke CH Jr. Safety and immunogenicity of attenuated dengue virus vaccines (Aventis Pasteur) in human volunteers. Vaccine. 2001. 19:3179–3188.
Article7. Edelman R, Wasserman SS, Bodison SA, Putnak RJ, Eckels KH, Tang D, Kanesa-Thasan N, Vaughn DW, Innis BL, Sun W. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003. 69:6 Suppl. 48–60.
Article8. Kitchener S, Nissen M, Nasveld P, Forrat R, Yoksan S, Lang J, Saluzzo JF. Immunogenicity and safety of two live-attenuated tetravalent dengue vaccine formulations in healthy Australian adults. Vaccine. 2006. 24:1238–1241.
Article9. Sun W, Cunningham D, Wasserman SS, Perry J, Putnak JR, Eckels KH, Vaughn DW, Thomas SJ, Kanesa-Thasan N, Innis BL, Edelman R. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naïve adults. Hum Vaccin. 2009. 5:33–40.
Article10. Kelly EP, Greene JJ, King AD, Innis BL. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine. 2000. 18:2549–2559.
Article11. Muné M, Rodríguez R, Ramírez R, Soto Y, Sierra B, Rodríguez Roche R, Marquez G, Garcia J, Guillén G, Guzmán MG. Carboxy-terminally truncated Dengue 4 virus envelope glycoprotein expressed in Pichia pastoris induced neutralizing antibodies and resistance to Dengue 4 virus challenge in mice. Arch Virol. 2003. 148:2267–2273.
Article12. Hermida L, Rodríguez R, Lazo L, Bernardo L, Silva R, Zulueta A, López C, Martín J, Valdés I, del Rosario D, Guillén G, Guzmán MG. A fragment of the envelope protein from dengue-1 virus, fused in two different sites of the meningococcal P64k protein carrier, induces a functional immune response in mice. Biotechnol Appl Biochem. 2004. 39:107–114.
Article13. Hermida L, Bernardo L, Martín J, Alvarez M, Prado I, López C, Sierra Bde L, Martínez R, Rodríguez R, Zulueta A, Pérez AB, Lazo L, Rosario D, Guillén G, Guzmán MG. A recombinant fusion protein containing the domain III of the dengue-2 envelope protein is immunogenic and protective in nonhuman primates. Vaccine. 2006. 24:3165–3171.
Article14. Blaney JE Jr, Hanson CT, Firestone CY, Hanley KA, Murphy BR, Whitehead SS. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am J Trop Med Hyg. 2004. 71:811–821.
Article15. Blaney JE Jr, Matro JM, Murphy BR, Whitehead SS. Recombinant, live-attenuated tetravalent dengue virus vaccine formulations induce a balanced, broad, and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005. 79:5516–5528.
Article16. Suzuki R, Winkelmann ER, Mason PW. Construction and characterization of a single-cycle chimeric flavivirus vaccine candidate that protects mice against lethal challenge with dengue virus type 2. J Virol. 2009. 83:1870–1880.
Article17. Azevedo AS, Yamamura AM, Freire MS, Trindade GF, Bonaldo M, Galler R, Alves AM. DNA vaccines against dengue virus type 2 based on truncate envelope protein or its domain III. PLoS One. 2011. 6:e20528.
Article18. Konishi E, Miyagawa Y. Balance of infection-enhancing and neutralizing antibodies induced by a dengue tetravalent DNA vaccine in a mouse model. Microbes Infect. 2011. 13:1091–1098.
Article19. Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011. 29:7221–7228.
Article20. Halstead SB, Deen J. The future of dengue vaccines. Lancet. 2002. 360:1243–1245.
Article21. Hombach J. Vaccines against dengue: a review of current candidate vaccines at advanced development stages. Rev Panam Salud Publica. 2007. 21:254–260.
Article22. Guy B, Almond JW. Towards a dengue vaccine: progress to date and remaining challenges. Comp Immunol Microbiol Infect Dis. 2008. 31:239–252.
Article23. Anderson KB, Gibbons RV, Edelman R, Eckels KH, Putnak RJ, Innis BL, Sun W. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J Infect Dis. 2011. 204:442–450.
Article24. Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010. 396:280–288.
Article25. Paris RM, Kim JH, Robb ML, Michael NL. Prime-boost immunization with poxvirus or adenovirus vectors as a strategy to develop a protective vaccine for HIV-1. Expert Rev Vaccines. 2010. 9:1055–1069.
Article26. Hill AV, Reyes-Sandoval A, O'Hara G, Ewer K, Lawrie A, Goodman A, Nicosia A, Folgori A, Colloca S, Cortese R, Gilbert SC, Draper SJ. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010. 6:78–83.
Article27. Vázquez-Blomquist D, Quintana D, Duarte CA. Modifiedvaccinia-virus-Ankara (MVA) priming and fowlpox-virus booster elicit a stronger CD8 T-cell response in mice against an HIV-1 epitope than does a DNA/poxvirus prime-booster approach. Biotechnol Appl Biochem. 2004. 39:313–318.28. Lu J, Wang C, Zhou Z, Zhang Y, Cao T, Shi C, Chen Z, Chen L, Cai C, Fan X. Immunogenicity and protective efficacy against murine tuberculosis of a prime-boost regimen with BCG and a DNA vaccine expressing ESAT-6 and Ag85A fusion protein. Clin Dev Immunol. 2011. 2011:617892.
Article29. Schneider J, Gilbert SC, Blanchard TJ, Hanke T, Robson KJ, Hannan CM, Becker M, Sinden R, Smith GL, Hill AV. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat Med. 1998. 4:397–402.
Article30. Rollier C, Verschoor EJ, Paranhos-Baccala G, Drexhage JA, Verstrepen BE, Berland JL, Himoudi N, Barnfield C, Liljestrom P, Lasarte JJ, Ruiz J, Inchauspe G, Heeney JL. Modulation of vaccine-induced immune responses to hepatitis C virus in rhesus macaques by altering priming before adenovirus boosting. J Infect Dis. 2005. 192:920–929.
Article31. Wu L, Kong WP, Nabel GJ. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J Virol. 2005. 79:8024–8031.
Article32. Barouch DH, Nabel GJ. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum Gene Ther. 2005. 16:149–156.
Article33. Tritel M, Stoddard AM, Flynn BJ, Darrah PA, Wu CY, Wille U, Shah JA, Huang Y, Xu L, Betts MR, Nabel GJ, Seder RA. Prime-boost vaccination with HIV-1 Gag protein and cytosine phosphate guanosine oligodeoxynucleotide, followed by adenovirus, induces sustained and robust humoral and cellular immune responses. J Immunol. 2003. 171:2538–2547.
Article34. Men R, Wyatt L, Tokimatsu I, Arakaki S, Shameem G, Elkins R, Chanock R, Moss B, Lai CJ. Immunization of rhesus monkeys with a recombinant of modified vaccinia virus Ankara expressing a truncated envelope glycoprotein of dengue type 2 virus induced resistance to dengue type 2 virus challenge. Vaccine. 2000. 18:3113–3122.
Article35. Eo SK, Lee S, Chun S, Rouse BT. Modulation of immunity against herpes simplex virus infection via mucosal genetic transfer of plasmid DNA encoding chemokines. J Virol. 2001. 75:569–578.
Article36. Walhout AJ, Temple GF, Brasch MA, Hartley JL, Lorson MA, van den, Vidal M. GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol. 2000. 328:575–592.37. Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985. 5:3403–3409.
Article38. Graziani-Bowering GM, Graham JM, Filion LG. A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes. J Immunol Methods. 1997. 207:157–168.
Article39. Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, Thiel A. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005. 11:1118–1124.
Article40. Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005. 11:1113–1117.
Article41. Aleyas AG, Han YW, George JA, Kim B, Kim K, Lee CK, Eo SK. Multifront assault on antigen presentation by Japanese encephalitis virus subverts CD8+ T cell responses. J Immunol. 2010. 185:1429–1441.
Article42. Lakhashe SK, Velu V, Sciaranghella G, Siddappa NB, Dipasquale JM, Hemashettar G, Yoon JK, Rasmussen RA, Yang F, Lee SJ, Montefiori DC, Novembre FJ, Villinger F, Amara RR, Kahn M, Hu SL, Li S, Li Z, Frankel FR, Robert-Guroff M, Johnson WE, Lieberman J, Ruprecht RM. Prime-boost vaccination with heterologous live vectors encoding SIV gag and multimeric HIV-1 gp160 protein: efficacy against repeated mucosal R5 clade C SHIV challenges. Vaccine. 2011. 29:5611–5622.
Article43. De Rosa SC, Thomas EP, Bui J, Huang Y, de Camp A, Morgan C, Kalams SA, Tomaras GD, Akondy R, Ahmed R, Lau CY, Graham BS, Nabel GJ, McElrath MJ. National Institute of Allergy and Infectious Diseases HIV Vaccine Trials Network: HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol. 2011. 187:3391–3401.
Article44. Kim SJ, Kim HK, Han YW, Aleyas AG, George JA, Yoon HA, Yoo DJ, Kim K, Eo SK. Multiple alternating immunizations with DNA vaccine and replication incompetent adenovirus expressing gB of pseudorabies virus protect animals against lethal virus challenge. J Microbiol Biotechnol. 2008. 18:1326–1334.45. DiNapoli JM, Yang L, Samal SK, Murphy BR, Collins PL, Bukreyev A. Respiratory tract immunization of non-human primates with a Newcastle disease virus-vectored vaccine candidate against Ebola virus elicits a neutralizing antibody response. Vaccine. 2010. 29:17–25.
Article46. Pan Z, Zhang X, Geng S, Fang Q, You M, Zhang L, Jiao X, Liu X. Prime-boost immunization using a DNA vaccine delivered by attenuated Salmonella enterica serovar typhimurium and a killed vaccine completely protects chickens from H5N1 highly pathogenic avian influenza virus. Clin Vaccine Immunol. 2010. 17:518–523.
Article47. Ding H, Tsai C, Gutirrez RA, Zhou F, Buchy P, Deubel V, Zhou P. Superior neutralizing antibody response and protection in mice vaccinated with heterologous DNA prime and virus like particle boost against HPAI H5N1 virus. PLoS One. 2011. 6:e16563.
Article48. Eo SK, Gierynska M, Kamar AA, Rouse BT. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J Immunol. 2001. 166:5473–5479.
Article49. Nguyen TV, Yuan L, Azevedo MS, Jeong KI, Gonzalez AM, Iosef C, Lovgren-Bengtsson K, Morein B, Lewis P, Saif LJ. High titers of circulating maternal antibodies suppress effector and memory B-cell responses induced by an attenuated rotavirus priming and rotavirus-like particle-immunostimulating complex boosting vaccine regimen. Clin Vaccine Immunol. 2006. 13:475–485.
Article50. Neeson P, Boyer J, Kumar S, Lewis MG, Mattias L, Veazey R, Weiner D, Paterson Y. A DNA prime-oral Listeria boost vaccine in rhesus macaques induces a SIV-specific CD8 T cell mucosal response characterized by high levels of alpha4beta7 integrin and an effector memory phenotype. Virology. 2006. 354:299–315.
Article51. Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol. 2010. 184:35–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo CTL Activity Induced by Prime-boost Vaccination using Recombinant Vaccinia Virus and DNA Vaccine Expressing Epitope Specific for CD8+ T Cells
- Influence of Immunity Induced at Priming Step on Mucosal Immunization of Heterologous Prime-Boost Regimens
- CD8+ T Cell-mediated Immunity Induced by Heterologous Prime-boost Vaccination Based on DNA Vaccine and Recombinant Vaccinia Virus Expressing Epitope
- DNA-mediated immunization of mice with plasmid encoding HBs antigen
- Protection of chicken against very virulent IBDV provided by in ovo priming with DNA vaccine and boosting with killed vaccine and the adjuvant effects of plasmid-encoded chicken interleukin-2 and interferon-gamma