Clin Exp Vaccine Res.
2013 Jul;2(2):76-82. 10.7774/cevr.2013.2.2.76.
Paths toward hepatitis B immunization in South Korea and Taiwan
- Affiliations
-
- 1Department of Sociology, National Cheng-chi University, Taipei, Taiwan. twchen@nccu.edu.tw
- KMID: 2167716
- DOI: http://doi.org/10.7774/cevr.2013.2.2.76
Abstract
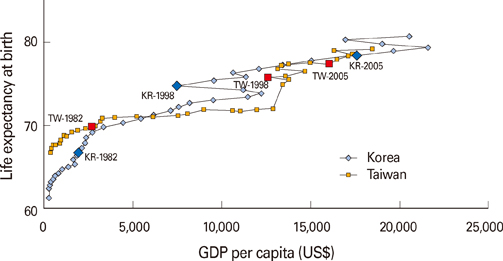
- South Korea and Taiwan have had similar experiences of economic development in the post-war era. The two societies have also successfully overcome the threat of liver cancer by using mass hepatitis B vaccinations. However, to reach their current states, they followed different directions, and experienced differing effects on their national health governance systems. In South Korea, vaccine production occurred prior to effectively introducing immunization programs. In contrast, Taiwan established an effective immunization program first. However, industrialization of vaccines against hepatitis B has failed. Taiwan has to import vaccines for domestic use. This article provides a contextual overview on the different methods South Korea and Taiwan have used to arrive at their modern status of hepatitis B immunization.
Keyword
MeSH Terms
Figure
Reference
-
1. Denis F, Levy-Bruhl D. Mass vaccination against hepatitis B: the French example. Curr Top Microbiol Immunol. 2006; 304:115–129.
Article2. Yang S, Khang YH, Harper S, Davey Smith G, Leon DA, Lynch J. Understanding the rapid increase in life expectancy in South Korea. Am J Public Health. 2010; 100:896–903.
Article3. Kim CY, Tilles JG. Purification and biophysical characterization of hepatitis B antigen. J Clin Invest. 1973; 52:1176–1186.
Article4. Han JY, Jung TW, Koh DK, Kim JH. A survey for changed control policies of hepatitis B in Republic of Korea. Korean J Pediatr Infect Dis. 2011; 18:124–134.
Article5. Hsu TC, Chow LP, Wei HY, Chen CL, Hsu ST. A controlled field trial for an evaluation of effectiveness of mouse-brain Japanese encephalitis vaccine. Taiwan Yi Xue Hui Za Zhi. 1971; 70:55–62.6. Hsu HM. History of hepatitis B in Taiwan. Epidemiol Bull (Taipei Taiwan). 1998; 14:82–91.7. Chen DS, Hsu NH, Sung JL, et al. A mass vaccination program in Taiwan against hepatitis B virus infection in infants of hepatitis B surface antigen-carrier mothers. JAMA. 1987; 257:2597–2603.
Article8. Chang MH, Chen CJ, Lai MS, et al. Taiwan Childhood Hepatoma Study Group. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med. 1997; 336:1855–1859.
Article9. Cha SH. The history of vaccination and current vaccination policies in Korea. Clin Exp Vaccine Res. 2012; 1:3–8.
Article10. Muraskin W. The war against hepatitis B: a history of the International Task Force on Hepatitis B Immunization. Philadelphia: University of Pennsylvania Press;1995. p. 23–65.11. Mahoney RT. Public-private partnership in the development of the hepatitis B vaccine in Korea: implications for developing countries. Sci Technol Soc. 2005; 10:129–140.
Article12. Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010; 53:20–28.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estimated impact of the national hepatitis B immunization program on acute viral hepatitis B among adolescents in Republic of Korea
- Another oral antiviral treatment, but still far away from hepatitis B virus cure
- Enhancing off-nucleos(t)ide analogue outcome predictions in chronic hepatitis B with time-varying hepatitis B core-related antigen
- Adult immunization
- Dyslipidemia in chronic hepatitis B patients on tenofovir alafenamide: Facts and puzzles