Ann Surg Treat Res.
2015 Apr;88(4):222-228. 10.4174/astr.2015.88.4.222.
De novo malignancy after liver transplantation: a single-center experience of 14 cases
- Affiliations
-
- 1Department of Hepatobiliary Surgery, Peking University People's Hospital, Beijing, China. gandanwk@vip.sina.com
- KMID: 2167021
- DOI: http://doi.org/10.4174/astr.2015.88.4.222
Abstract
- PURPOSE
The aim of this study is to evaluate the incidence of de novo malignancy after liver transplantation (LT) and compare with those among the general Chinese population.
METHODS
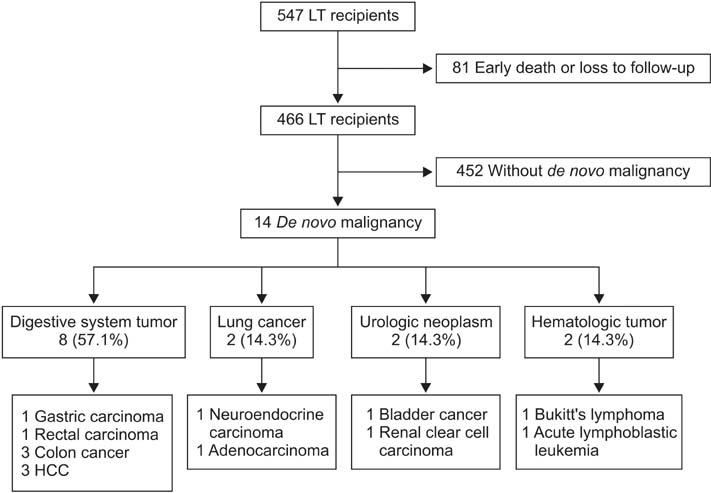
A total of 466 patients who had a minimum follow-up time of 6 months were enrolled in the study. All data of medical records and follow up were retrospectively reviewed.
RESULTS
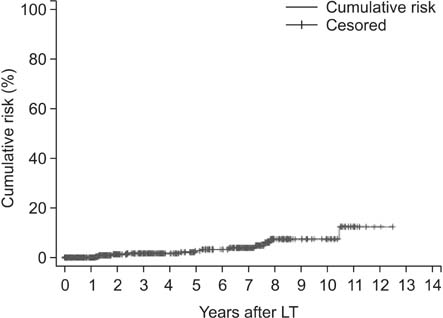
The incidence rate of de novo malignancy was 3.0% (14 in 466 patients). The median elapsed time from transplant to the diagnosis of de novo malignancy was 42 months (range, 6 to 106 months). The cumulative risk for development of de novo malignancy was 1.6%, 2.7%, and 8.2% at 3, 5 and 10 years after LT, respectively. The patients were all male. The types of de novo tumors included digestive system tumor (8 in 14), lung cancer (2 in 14), urologic neoplasm (2 in 14), and hematologic malignant tumor (2 in 14). Over a mean follow-up of 24 months after diagnosis of de novo malignancy, 7 patients (50.0%) died; the overall 5-year patient survival rate was 54.5%. The relative risk of malignancy following LT was 9.5 folds higher than the general Chinese population.
CONCLUSION
The relative risk of malignancy following LT was much higher than the general Chinese population. Digestive system tumor is the most common type of de novo malignancy after LT in China.
MeSH Terms
Figure
Cited by 1 articles
-
Surveillance and management of de novo malignancy after liver transplantation
Woo-Hyoung Kang
Ann Liver Transplant. 2024;4(1):10-15. doi: 10.52604/alt.24.0006.
Reference
-
1. Kim JM, Kwon CH, Yun IJ, Lee KW, Yu HC, Suh KS, et al. A multicenter experience with generic mycophenolate mofetil conversion in stable liver transplant recipients. Ann Surg Treat Res. 2014; 86:192–198.2. Kim JD, Choi DL, Han YS. The paracholedochal vein: a feasible option as portal inflow in living donor liver transplantation. Ann Surg Treat Res. 2014; 87:47–50.3. Sapisochin G, Bilbao I, Dopazo C, Castells L, Lazaro JL, Rodriguez R, et al. Evolution and management of de novo neoplasm post-liver transplantation: a 20-year experience from a single European centre. Hepatol Int. 2011; 5:707–715.4. Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of the US National Transplant Database. Transplantation. 2012; 94:990–998.5. Hegab B, Khalaf H, Allam N, Azzam A, Al Khail FA, Al-hamoudi W, et al. De novo malignancies after liver transplantation: a single-center experience. Ann Saudi Med. 2012; 32:355–358.6. Park HW, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. De novo malignancies after liver transplantation: incidence comparison with the Korean cancer registry. Transplant Proc. 2012; 44:802–805.7. Penn I. Occurrence of cancers in immunosuppressed organ transplant recipients. Clin Transpl. 1998; 147–158.8. Penn I. Post-transplant malignancy: the role of immunosuppression. Drug Saf. 2000; 23:101–113.9. Soltys KA, Mazariegos GV, Squires RH, Sindhi RK, Anand R. SPLIT Research Group. Late graft loss or death in pediatric liver transplantation: an analysis of the SPLIT database. Am J Transplant. 2007; 7:2165–2171.10. Tiao GM, Bobey N, Allen S, Nieves N, Alonso M, Bucuvalas J, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr. 2005; 146:204–211.11. Belloni-Fortina A, Piaserico S, Bordignon M, Gambato M, Senzolo M, Russo FP, et al. Skin cancer and other cutaneous disorders in liver transplant recipients. Acta Derm Venereol. 2012; 92:411–415.12. Zhu ZJ, Li L, Zhang YM, Zheng H, Jiang WT, Zhang JJ, et al. The diagnosis and treatment of de novo malignancy after liver transplantion. Chin J Oncol. 2007; 29:237–238.13. Zhang T, Fu BS, Yi HM, Yi SH, Xu C, Wang GS, et al. The clinical analysis of de novo malignant tumors after liver transplantation: report of four cases. Chin J Organ Transpl. 2010; 31:356–359.14. Zhang T, Zhang YQ, Fu BS, Yang Y, Cai CJ, Lu MQ, et al. De novo lung cancer after liver transplantation: report of one case and literature review. Organ Transpl. 2010; 1:281–286.15. Lv Y, Hu LS, Liu C, Yu L, Liu XM, Wang B, et al. Suffering from secondary laryngeal neuroendocrine carcinoma after liver retransplantation, one case report. J Mod Oncol. 2008; 16:1210–1211.16. Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013; 25:10–21.17. Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L, Altaca G, Guarrera JV, et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation. 2000; 69:781–789.18. Boin I, Leonardi MI, Stucchi RB, Ataide EC, Almeida JR, Barros RH, et al. De novo posttransplantation nonlymphoproliferative malignancies in liver transplant recipients. Transplant Proc. 2007; 39:3284–3286.19. Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, et al. Increased cancer risk after liver transplantation: a populationbased study. J Hepatol. 2001; 34:84–91.20. Romero-Vargas ME, Flores-Cortes M, Valera Z, Gomez-Bravo MA, Barrera-Pulido L, Pareja-Ciuro F, et al. Cancers of new appearance in liver transplant recipients: incidence and evolution. Transplant Proc. 2006; 38:2508–2510.21. Jain AB, Yee LD, Nalesnik MA, Youk A, Marsh G, Reyes J, et al. Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation. 1998; 66:1193–1200.22. Guthery SL, Heubi JE, Bucuvalas JC, Gross TG, Ryckman FC, Alonso MH, et al. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003; 75:987–993.23. Euvrard S, Kanitakis J. Skin cancers after liver transplantation: what to do. J Hepatol. 2006; 44:27–32.24. Peyregne V, Ducerf C, Adham M, de la Roche E, Berthoux N, Bancel B, et al. De novo cancer after orthotopic liver transplantation. Transplant Proc. 1998; 30:1484–1485.25. Fei JG, Chen LZ, Zhao JQ, Wang CX, Qiu J, Deng SX, et al. Characteristics and risk factors of malignant tumor in kidney transplantation recipients. China Oncol. 2008; 18:223–226.26. Fan Y, Shi BY, Chang JY, Bo HW, Wang Z, Ou YJ, et al. Analysis on the occurrence of malignant tumors after kidney transplantation. Med J Chin People Lib Army. 2007; 32:529–530.27. Terhorst D, Drecoll U, Stockfleth E, Ulrich C. Organ transplant recipients and skin cancer: assessment of risk factors with focus on sun exposure. Br J Dermatol. 2009; 161:Suppl 3. 85–89.28. Campistol JM. Minimizing the risk of posttransplant malignancy. Transplant Proc. 2008; 40:10 Suppl. S40–S43.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- De novo gastric cancer after liver transplantation: A review of the Asian experience
- Surveillance and management of de novo malignancy after liver transplantation
- Pancreaticoduodenectomy for de novo ampulla of Vater cancer 15 years after living donor liver transplantation: Report of a case
- Association of human leukocyte antigen homozygosity and de novo malignancies in kidney transplant recipients
- Prophylaxis for Hepatitis B Core Antibody-Positive Donors after Liver Transplantation