Conversion and Data Quality Assessment of Electronic Health Record Data at a Korean Tertiary Teaching Hospital to a Common Data Model for Distributed Network Research
- Affiliations
-
- 1Department of Biomedical Informatics, Ajou University School of Medicine, Suwon, Korea. veritas@ajou.ac.kr
- 2Observational Health Data Sciences and Informatics, New York, NY, USA.
- 3Department of Nursing Science, Dongyang University, Yeongju, Korea.
- 4Mibyeong Research Center, Korea Institute of Oriental Medicine, Daejeon, Korea.
- 5Global Epidemiology, Janssen Research and Development LLC, Titusville, NJ, USA.
- KMID: 2166917
- DOI: http://doi.org/10.4258/hir.2016.22.1.54
Abstract
OBJECTIVES
A distributed research network (DRN) has the advantages of improved statistical power, and it can reveal more significant relationships by increasing sample size. However, differences in data structure constitute a major barrier to integrating data among DRN partners. We describe our experience converting Electronic Health Records (EHR) to the Observational Health Data Sciences and Informatics (OHDSI) Common Data Model (CDM).
METHODS
We transformed the EHR of a hospital into Observational Medical Outcomes Partnership (OMOP) CDM ver. 4.0 used in OHDSI. All EHR codes were mapped and converted into the standard vocabulary of the CDM. All data required by the CDM were extracted, transformed, and loaded (ETL) into the CDM structure. To validate and improve the quality of the transformed dataset, the open-source data characterization program ACHILLES was run on the converted data.
RESULTS
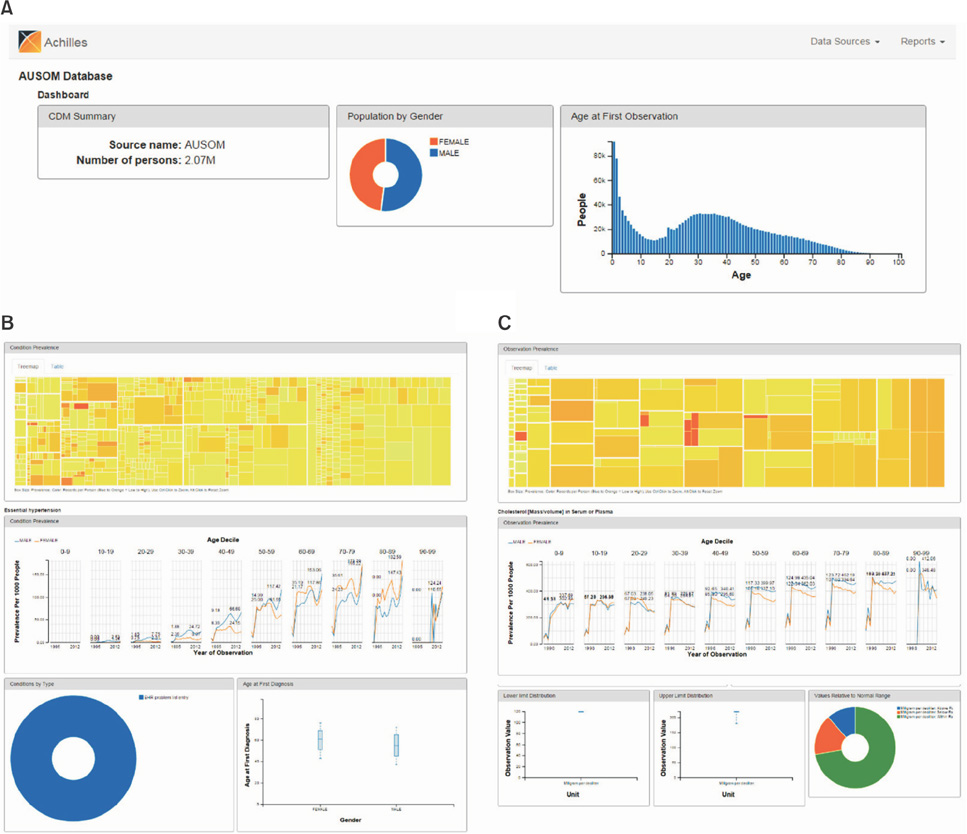
Patient, drug, condition, procedure, and visit data from 2.07 million patients who visited the subject hospital from July 1994 to November 2014 were transformed into the CDM. The transformed dataset was named the AUSOM. ACHILLES revealed 36 errors and 13 warnings in the AUSOM. We reviewed and corrected 28 errors. The summarized results of the AUSOM processed with ACHILLES are available at http://ami.ajou.ac.kr:8080/.
CONCLUSIONS
We successfully converted our EHRs to a CDM and were able to participate as a data partner in an international DRN. Converting local records in this manner will provide various opportunities for researchers and data holders.
Keyword
MeSH Terms
Figure
Cited by 5 articles
-
Treatment Patterns of Type 2 Diabetes Assessed Using a Common Data Model Based on Electronic Health Records of 2000–2019
Kyung Ae Lee, Heung Yong Jin, Yu Ji Kim, Yong-Jin Im, Eun-Young Kim, Tae Sun Park
J Korean Med Sci. 2021;36(36):e230. doi: 10.3346/jkms.2021.36.e230.Anesthesiologist’s Perspective on the Standardization of Clinical Terminology in Electronic Health Records
Kyoung Ok Kim
Healthc Inform Res. 2021;27(4):350-351. doi: 10.4258/hir.2021.27.4.350.Computer-based clinical coding activity analysis for neurosurgical terms
Jong Hyuk Lee, Jung Hwan Lee, Wooseok Ryu, Byung Kwan Choi, In Ho Han, Chang Min Lee
Yeungnam Univ J Med. 2019;36(3):225-230. doi: 10.12701/yujm.2019.00220.Applying the OMOP Common Data Model to Facilitate Benefit-Risk Assessments of Medicinal Products Using Real-World Data from Singapore and South Korea
Hui Xing Tan, Desmond Chun Hwee Teo, Dongyun Lee, Chungsoo Kim, Jing Wei Neo, Cynthia Sung, Haroun Chahed, Pei San Ang, Doreen Su Yin Tan, Rae Woong Park, Sreemanee Raaj Dorajoo
Healthc Inform Res. 2022;28(2):112-122. doi: 10.4258/hir.2022.28.2.112.Utility of Treatment Pattern Analysis Using a Common Data Model: A Scoping Review
Eun-Gee Park, Min Jung Kim, Jinseo Kim, Kichul Shin, Borim Ryu
Healthc Inform Res. 2025;31(1):4-15. doi: 10.4258/hir.2025.31.1.4.
Reference
-
1. Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network: improving the evidence of medical-product safety. N Engl J Med. 2009; 361(7):645–647.
Article2. Observational Health Data Sciences and Informatics. Analytic tools [Internet]. [place unknown]: Observational Health Data Sciences and Informatics;c2015. cited at 2015 Dec 1. Available from: http://www.ohdsi.org/analytic-tools/.3. Mini-Sentinel project [Internet]. [place unknown]: Mini-Sentinel Coordinating Center;c2014. cited at 2015 Dec 1. Available from: http://www.mini-sentinel.org/.4. Observational Medical Outcomes Partnership. OMOP Common Data Model [Internet]. [place unknown]: Observational Medical Outcomes Partnership;2013. cited at 2015 Dec 1. Available from: http://omop.org/CDM.5. Boyce RD, Ryan PB, Noren GN, Schuemie MJ, Reich C, Duke J, et al. Bridging islands of information to establish an integrated knowledge base of drugs and health outcomes of interest. Drug Saf. 2014; 37(8):557–567.
Article6. Yoon D, Chang BC, Kang SW, Bae H, Park RW. Adoption of electronic health records in Korean tertiary teaching and general hospitals. Int J Med Inform. 2012; 81:196–203.
Article7. Madigan D, Ryan PB, Schuemie M, Stang PE, Overhage JM, Hartzema AG, et al. Evaluating the impact of database heterogeneity on observational study results. Am J Epidemiol. 2013; 178(4):645–651.
Article8. Dodd S. Debating the evidence: oral contraceptives containing drospirenone and risk of blood clots. Curr Drug Saf. 2011; 6(3):132–133.
Article9. Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 2012; 125(20):2520–2544.
Article10. Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010; 48:6 Suppl. S45–S51.11. Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, et al. Design considerations, architecture, and use of the Mini-Sentinel distributed data system. Pharmacoepidemiol Drug Saf. 2012; 21:Suppl 1. 23–31.
Article12. Matcho A, Ryan P, Fife D, Reich C. Fidelity assessment of a clinical practice research datalink conversion to the OMOP common data model. Drug Saf. 2014; 37(11):945–959.
Article13. Coloma PM, Schuemie MJ, Trifiro G, Gini R, Herings R, Hippisley-Cox J, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011; 20(1):1–11.
Article14. Rijnbeek PR. Converting to a common data model: what is lost in translation? Commentary on "fidelity assessment of a clinical practice research datalink conversion to the OMOP common data model". Drug Saf. 2014; 37(11):893–896.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessing the Quality of Structured Data Entry for the Secondary Use of Electronic Medical Records
- Availability of nursing data in an electronic nursing record system for a development of a risk assessment tool for pressure ulcers
- Establishment of an International Evidence Sharing Network Through Common Data Model for Cardiovascular Research
- Needs Assessment for Functionalities in Electronic Health Record Systems in General Hospitals
- Japanese EMRs and IT in Medicine: Expansion, Integration, and Reuse of Data