J Bone Metab.
2016 May;23(2):79-83. 10.11005/jbm.2016.23.2.79.
Which Bisphosphonate? It's the Compliance!: Decision Analysis
- Affiliations
-
- 1Department of Internal Medicine, Center for Thyroid Cancer, National Cancer Center, Goyang, Korea.
- 2Department of Orthopaedic Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. ykleemd@gmail.com
- 3Department of Orthopaedic Surgery, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2165121
- DOI: http://doi.org/10.11005/jbm.2016.23.2.79
Abstract
- BACKGROUND
The best options of several bisphosphonates for prevention of osteoporotic fractures in postmenopausal women remain controversial. We determined which bisphosphonate provides better efficacy in prevention of osteoporotic fractures using a decision analysis tool, in terms of quality of life.
METHODS
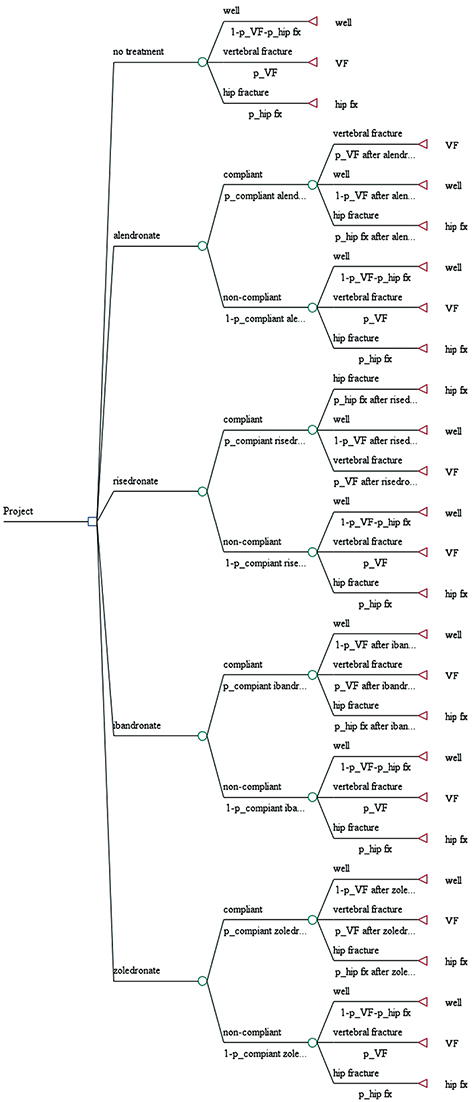
A decision analysis model was constructed containing final outcome score and the probability of vertebral and hip fracture within 1 year. Final outcome was defined as health-related quality of life, and was used as an utility in the decision tree. Probabilities were obtained by literature review, and health-related quality of life was evaluated by consensus committee. A roll back tool was used to determine the best bisphosphonate, and sensitivity analysis was performed to compensate for decision model uncertainty.
RESULTS
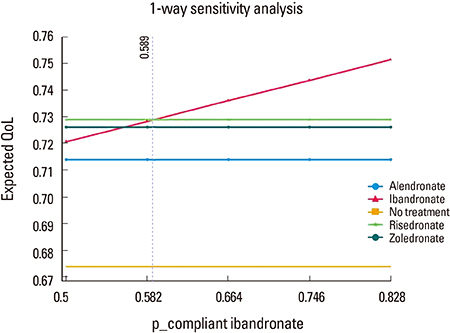
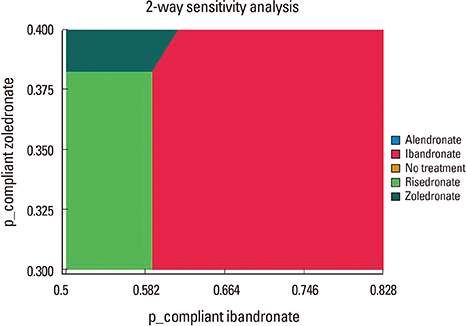
The decision model favored bisphosphonate with higher compliance in terms of quality of life. In one-way sensitivity analysis, ibandronate was more beneficial than the others, when probability of compliance on ibandronate was above 0.589.
CONCLUSIONS
In terms of quality of life, the decision analysis model showed that compliance was most important for patients in real world, regardless of type of bisphosphonate.
MeSH Terms
Figure
Cited by 3 articles
-
The Efficacy of Bisphosphonates for Prevention of Osteoporotic Fracture: An Update Meta-analysis
Ji-Hye Byun, Sunmee Jang, Sumin Lee, Suyeon Park, Hyun Koo Yoon, Byung-Ho Yoon, Yong-Chan Ha
J Bone Metab. 2017;24(1):37-49. doi: 10.11005/jbm.2017.24.1.37.Osteoblasts Are the Centerpiece of the Metastatic Bone Microenvironment
Hyo Min Jeong, Sun Wook Cho, Serk In Park
Endocrinol Metab. 2016;31(4):485-492. doi: 10.3803/EnM.2016.31.4.485.Medications for osteoporotic pain
Sang Wook Shin
Korean J Pain. 2017;30(2):85-85. doi: 10.3344/kjp.2017.30.2.85.
Reference
-
1. Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996; 348:1535–1541.
Article2. Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999; 282:1344–1352.
Article3. Chesnut CH 3rd, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004; 19:1241–1249.
Article4. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356:1809–1822.
Article5. Brauer CA, Bozic KJ. Using observational data for decision analysis and economic analysis. J Bone Joint Surg Am. 2009; 91:Suppl 3. 73–79.
Article6. Bernstein J. Decision analysis. J Bone Joint Surg Am. 1997; 79:1404–1414.
Article7. Park C, Ha YC, Jang S, et al. The incidence and residual lifetime risk of osteoporosis-related fractures in Korea. J Bone Miner Metab. 2011; 29:744–751.
Article8. Lee YK, Nho JH, Ha YC, et al. Persistence with intravenous zoledronate in elderly patients with osteoporosis. Osteoporos Int. 2012; 23:2329–2333.
Article9. Cramer JA, Amonkar MM, Hebborn A, et al. Compliance and persistence with bisphosphonate dosing regimens among women with postmenopausal osteoporosis. Curr Med Res Opin. 2005; 21:1453–1460.
Article10. Cooper A, Drake J, Brankin E. Treatment persistence with once-monthly ibandronate and patient support vs. once-weekly alendronate: results from the PERSIST study. Int J Clin Pract. 2006; 60:896–905.
Article11. Russell RG, Watts NB, Ebetino FH, et al. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19:733–759.
Article12. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009; 122:S14–S21.
Article13. Siris ES, Selby PL, Saag KG, et al. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am J Med. 2009; 122:S3–S13.
Article14. Hadji P, Claus V, Ziller V, et al. GRAND: the German retrospective cohort analysis on compliance and persistence and the associated risk of fractures in osteoporotic women treated with oral bisphosphonates. Osteoporos Int. 2012; 23:223–231.
Article15. Landfeldt E, Ström O, Robbins S, et al. Adherence to treatment of primary osteoporosis and its association to fractures--the Swedish Adherence Register Analysis (SARA). Osteoporos Int. 2012; 23:433–443.
Article16. Kamatari M, Koto S, Ozawa N, et al. Factors affecting long-term compliance of osteoporotic patients with bisphosphonate treatment and QOL assessment in actual practice: alendronate and risedronate. J Bone Miner Metab. 2007; 25:302–309.
Article17. Kertes J, Dushenat M, Vesterman JL, et al. Factors contributing to compliance with osteoporosis medication. Isr Med Assoc J. 2008; 10:207–213.18. Weycker D, Macarios D, Edelsberg J, et al. Compliance with osteoporosis drug therapy and risk of fracture. Osteoporos Int. 2007; 18:271–277.
Article19. Cramer JA, Gold DT, Silverman SL, et al. A systematic review of persistence and compliance with bisphosphonates for osteoporosis. Osteoporos Int. 2007; 18:1023–1031.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can Alarming Improve Compliance with Weekly Bisphosphonate in Patients with Osteoporosis?
- The clinical decision analysis using decision tree
- Impact on Bisphosphonate Persistence and Compliance: Daily Postprandial Administration
- Insufficiency Fracture of Ipsilateral Femur Neck in Patient Treated with Long Term Bisphosphonate Treatment: A Case Report
- Bisphosphonate: An Invaluable Medication or Abandoned Acid?