J Korean Med Sci.
2015 Aug;30(8):1121-1128. 10.3346/jkms.2015.30.8.1121.
Clinical Features and Prognosis of Invasive Pulmonary Aspergillosis in Korean Children with Hematologic/Oncologic Diseases
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. pedjsyoon@catholic.ac.kr
- 2Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Catholic Blood and Marrow Transplantation Center, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 4Division of Infectious Diseases, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 5Department of Radiology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2164507
- DOI: http://doi.org/10.3346/jkms.2015.30.8.1121
Abstract
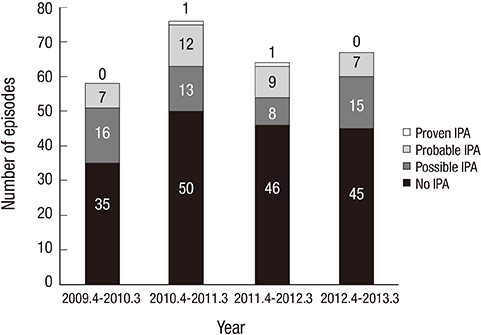
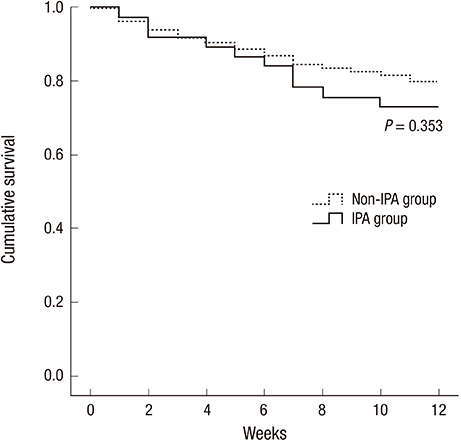
- Invasive pulmonary aspergillosis (IPA) is the most frequent form of invasive fungal diseases in immunocompromised patients. However, there are only a few studies on IPA in immunocompromised children in Korea. This study was designed to characterize IPA in Korean children with hematologic/oncologic diseases. Medical records of children with hematologic/oncologic diseases receiving antifungal therapy were reviewed. The enrolled children were divided into the IPA group (proven and probable IPA) and non-IPA group, and the clinical characteristics and prognosis were compared between the two groups. During the study period, 265 courses of antifungal therapy were administered to 166 children. Among them, two (0.8%) episodes of proven IPA, 35 (13.2%) of probable IPA, and 52 (19.6%) of possible IPA were diagnosed. More children in the IPA group suffered from neutropenia lasting for more than two weeks (51.4% vs. 21.9%, P<0.001) and showed halo signs on the chest computed tomography (78.4% vs. 40.7%, P<0.001) than in the non-IPA group. No other clinical factors showed significant differences between the two groups. Amphotericin B deoxycholate was administered as a first line antifungal agent in 33 (89.2%) IPA group episodes, and eventually voriconazole was administered in 27 (73.0%) episodes. Ten (27.0%) children in the IPA group died within 12 weeks of antifungal therapy. In conclusion, early use of chest computed tomography to identify halo signs in immunocompromised children who are expected to have prolonged neutropenia can be helpful for early diagnosis of IPA and improving prognosis of children with IPA.
MeSH Terms
-
Antifungal Agents/*therapeutic use
Child
Child Health/statistics & numerical data
Comorbidity
Female
Hematologic Diseases/*mortality
Humans
Incidence
Invasive Pulmonary Aspergillosis/*diagnosis/drug therapy/*mortality
Male
Neoplasms/*mortality
Prognosis
Republic of Korea/epidemiology
Risk Factors
Survival Rate
Tomography, X-Ray Computed/statistics & numerical data
Treatment Outcome
Antifungal Agents
Figure
Reference
-
1. Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, Corey L, Boeckh M. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000; 96:2055–2061.2. Kaya Z, Gursel T, Kocak U, Aral YZ, Kalkanci A, Albayrak M. Invasive fungal infections in pediatric leukemia patients receiving fluconazole prophylaxis. Pediatr Blood Cancer. 2009; 52:470–475.3. Mor M, Gilad G, Kornreich L, Fisher S, Yaniv I, Levy I. Invasive fungal infections in pediatric oncology. Pediatr Blood Cancer. 2011; 56:1092–1097.4. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001; 32:358–366.5. Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore). 2000; 79:250–260.6. Groll AH, Kurz M, Schneider W, Witt V, Schmidt H, Schneider M, Schwabe D. Five-year-survey of invasive aspergillosis in a paediatric cancer centre. Epidemiology, management and long-term survival. Mycoses. 1999; 42:431–442.7. Abbasi S, Shenep JL, Hughes WT, Flynn PM. Aspergillosis in children with cancer: A 34-year experience. Clin Infect Dis. 1999; 29:1210–1219.8. Babor F, Schuster F, Mackenzie C, Meisel R, Schaper J, Sabir H, Siepermann M, Wessalowski R, Janßen G, Borkhardt A, et al. Invasive aspergillosis in pediatric oncology patients: a rare event with poor prognosis--case analysis to plan better targeted prophylactic or therapeutic measurement. Klin Padiatr. 2012; 224:160–165.9. Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, Kern WV, Marr KA, Ribaud P, Lortholary O, et al. Invasive Fungal Infections Group of the European Organisation for Research and Treatment of Cancer and the Global Aspergillus Study Group. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347:408–415.10. Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis. 2009; 48:265–273.11. Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012; 65:453–464.12. Choi SH, Kang ES, Eo H, Yoo SY, Kim JH, Yoo KH, Sung KW, Koo HH, Kim YJ. Aspergillus galactomannan antigen assay and invasive aspergillosis in pediatric cancer patients and hematopoietic stem cell transplant recipients. Pediatr Blood Cancer. 2013; 60:316–322.13. Kim SH, Moon SM, Han SH, Chung JW, Moon SY, Lee MS, Choo EJ, Choi YH, Kim SW, Bae IG, et al. Epidemiology and clinical outcomes of invasive pulmonary aspergillosis: a nationwide multicenter study in Korea. Infect Chemother. 2012; 44:282–288.14. Kwon JC, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH, Kim YJ, Lee S, Kim HJ, et al. Prognosis of invasive pulmonary aspergillosis in patients with hematologic diseases in Korea. Tuberc Respir Dis (Seoul). 2012; 72:284–292.15. Nivoix Y, Velten M, Letscher-Bru V, Moghaddam A, Natarajan-Amé S, Fohrer C, Lioure B, Bilger K, Lutun P, Marcellin L, et al. Factors associated with overall and attributable mortality in invasive aspergillosis. Clin Infect Dis. 2008; 47:1176–1184.16. Caillot D, Casasnovas O, Bernard A, Couaillier JF, Durand C, Cuisenier B, Solary E, Piard F, Petrella T, Bonnin A, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997; 15:139–147.17. Groll AH, Castagnola E, Cesaro S, Dalle JH, Engelhard D, Hope W, Roilides E, Styczynski J, Warris A, Lehrnbecher T. Fourth European Conference on Infections in Leukaemia. Infectious Diseases Working Party of the European Group for Blood Marrow Transplantation (EBMT-IDWP). Infectious Diseases Group of the European Organisation for Research and Treatment of Cancer (EORTC-IDG). International Immunocompromised Host Society (ICHS). European Leukaemia Net (ELN). Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or allogeneic haemopoietic stem-cell transplantation. Lancet Oncol. 2014; 15:e327–e340.18. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR. Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011; 52:e56–e93.19. Caselli D, Cesaro S, Ziino O, Ragusa P, Pontillo A, Pegoraro A, Santoro N, Zanazzo G, Poggi V, Giacchino M, et al. A prospective, randomized study of empirical antifungal therapy for the treatment of chemotherapy-induced febrile neutropenia in children. Br J Haematol. 2012; 158:249–255.20. Cesaro S, Pagano L, Caira M, Carraro F, Luciani M, Russo D, Colombini A, Morello W, Viale P, Rossi G, et al. Hema-e-chart Group. A prospective, multicentre survey on antifungal therapy in neutropenic paediatric haematology patients. Mycoses. 2013; 56:21–25.21. des Champs-Bro B, Leroy-Cotteau A, Mazingue F, Pasquier F, François N, Corm S, Lemaitre L, Poulain D, Yakoub-Agha I, Alfandari S, et al. Invasive fungal infections: epidemiology and analysis of antifungal prescriptions in onco-haematology. J Clin Pharm Ther. 2011; 36:152–160.22. Crassard N, Hadden H, Piens MA, Pondarré C, Hadden R, Galambrun C, Pracros JP, Souillet G, Basset T, Berthier JC, et al. Invasive aspergillosis in a paediatric haematology department: a 15-year review. Mycoses. 2008; 51:109–116.23. Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, Prasad P, Steinbach WJ. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. 2008; 121:e1286–e1294.24. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008; 46:1813–1821.25. Lee DG, Kim SH, Kim SY, Kim CJ, Min CK, Park WB, Park YJ, Song YG, Jang JS, Jang JH, et al. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Infect Chemother. 2011; 43:258–321.26. Segal BH, Herbrecht R, Stevens DA, Ostrosky-Zeichner L, Sobel J, Viscoli C, Walsh TJ, Maertens J, Patterson TF, Perfect JR, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008; 47:674–683.27. Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002; 100:4358–4366.28. Zaoutis TE, Heydon K, Chu JH, Walsh TJ, Steinbach WJ. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics. 2006; 117:e711–e716.29. Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, Ibatici A, Del Bono V, Bacigalupo A, Viscoli C. Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant. 2009; 44:361–370.30. Steinbach WJ. Invasive aspergillosis in pediatric patients. Curr Med Res Opin. 2010; 26:1779–1787.31. Caillot D, Couaillier JF, Bernard A, Casasnovas O, Denning DW, Mannone L, Lopez J, Couillault G, Piard F, Vagner O, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001; 19:253–259.32. Hayden R, Pounds S, Knapp K, Petraitiene R, Schaufele RL, Sein T, Walsh TJ. Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis. Pediatr Infect Dis J. 2008; 27:815–819.33. Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010; 50:1091–1100.34. Desai R, Ross LA, Hoffman JA. The role of bronchoalveolar lavage galactomannan in the diagnosis of pediatric invasive aspergillosis. Pediatr Infect Dis J. 2009; 28:283–286.35. Hsu LY, Ding Y, Phua J, Koh LP, Chan DS, Khoo KL, Tambyah PA. Galactomannan testing of bronchoalveolar lavage fluid is useful for diagnosis of invasive pulmonary aspergillosis in hematology patients. BMC Infect Dis. 2010; 10:44.36. Becker MJ, de Marie S, Fens MH, Verbrugh HA, Bakker-Woudenberg IA. Effect of amphotericin B treatment on kinetics of cytokines and parameters of fungal load in neutropenic rats with invasive pulmonary aspergillosis. J Antimicrob Chemother. 2003; 52:428–434.37. Greene RE, Schlamm HT, Oestmann JW, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007; 44:373–379.38. Bowden R, Chandrasekar P, White MH, Li X, Pietrelli L, Gurwith M, van Burik JA, Laverdiere M, Safrin S, Wingard JR. A double-blind, randomized, controlled trial of amphotericin B colloidal dispersion versus amphotericin B for treatment of invasive aspergillosis in immunocompromised patients. Clin Infect Dis. 2002; 35:359–366.39. Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, Pappas P, Seibel N, Greenberg RN, Dummer S, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999; 340:764–771.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulmonary Resection for Invasive Pulmonary Aspergillosis in Hematological Malignancy Patients
- A Case of Acute Interstitial Pneumonia with Invasive Pulmonary Aspergillosis
- Surgical Management of Invasive Pulmonary Aspergillosis in Hemtologic Malignancy Patients: Report of 2 cases
- A Case of chronic necrotizing pulmonary aspergillosis with pulmonary artery aneurysm
- A Case of Endobronchial Aspergillosis Completely Obstructing Lobar Bronchus