Diabetes Metab J.
2016 Apr;40(2):154-160. 10.4093/dmj.2016.40.2.154.
The Level of Autoantibodies Targeting Eukaryote Translation Elongation Factor 1 α1 and Ubiquitin-Conjugating Enzyme 2L3 in Nondiabetic Young Adults
- Affiliations
-
- 1Department of Systems Immunology, College of Biomedical Science and the Institute of Bioscience and Biotechnology, Kangwon National University, Chuncheon, Korea. kmkim@kangwon.ac.kr
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. bokyungkoomd@gmail.com
- 3Department of New Biology, Daegu Gyeongbuk Institute of Science and Technology and Institute for Basic Science, Daegu, Korea.
- 4Molecular Medicine and Biopharmaceutical Sciences, Seoul National University College of Medicine and Pharmacy, Seoul, Korea.
- 5Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2162087
- DOI: http://doi.org/10.4093/dmj.2016.40.2.154
Abstract
- BACKGROUND
The prevalence of novel type 1 diabetes mellitus (T1DM) antibodies targeting eukaryote translation elongation factor 1 alpha 1 autoantibody (EEF1A1-AAb) and ubiquitin-conjugating enzyme 2L3 autoantibody (UBE2L3-AAb) has been shown to be negatively correlated with age in T1DM subjects. Therefore, we aimed to investigate whether age affects the levels of these two antibodies in nondiabetic subjects.
METHODS
EEF1A1-AAb and UBE2L3-AAb levels in nondiabetic control subjects (n=150) and T1DM subjects (n=101) in various ranges of age (18 to 69 years) were measured using an enzyme-linked immunosorbent assay. The cutoff point for the presence of each autoantibody was determined based on control subjects using the formula: [mean absorbance+3×standard deviation].
RESULTS
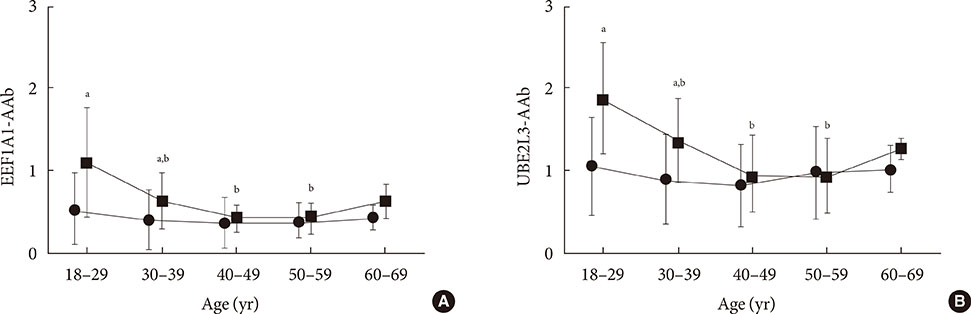
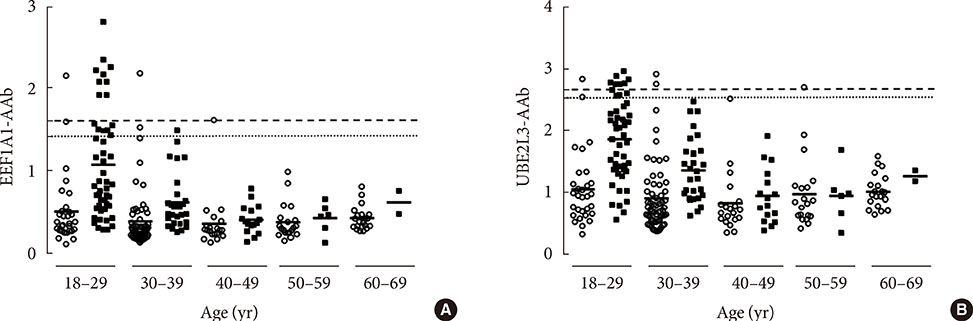
In nondiabetic subjects, there were no significant correlations between age and EEF1A1-AAb and UBE2L3-AAb levels. However, there was wide variation in EEF1A1-AAb and UBE2L3-AAb levels among control subjects <40 years old; the prevalence of both EEF1A1-AAb and UBE2L3-AAb in these subjects was 4.4%. When using cutoff points determined from the control subjects <40 years old, the prevalence of both autoantibodies in T1DM subjects was decreased (EEFA1-AAb, 15.8% to 8.9%; UBE2L3-AAb, 10.9% to 7.9%) when compared to the prevalence using the cutoff derived from the totals for control subjects.
CONCLUSION
There was no association between age and EEF1A1-AAb or UBE2L3-AAb levels in nondiabetic subjects. However, the wide variation in EEF1A1-AAb and UBE2L3-AAb levels apparent among the control subjects <40 years old should be taken into consideration when determining the cutoff reference range for the diagnosis of T1DM.
Keyword
MeSH Terms
Figure
Reference
-
1. Verge CF, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson RA, Chase HP, Eisenbarth GS. Prediction of type I diabetes in first-degree relatives using a combination of insulin, GAD, and ICA512bdc/IA-2 autoantibodies. Diabetes. 1996; 45:926–933.2. Greenbaum CJ, Sears KL, Kahn SE, Palmer JP. Relationship of beta-cell function and autoantibodies to progression and nonprogression of subclinical type 1 diabetes: follow-up of the Seattle Family Study. Diabetes. 1999; 48:170–175.3. Zampetti S, Capizzi M, Spoletini M, Campagna G, Leto G, Cipolloni L, Tiberti C, Bosi E, Falorni A, Buzzetti R. NIRAD Study Group. GADA titer-related risk for organ-specific autoimmunity in LADA subjects subdivided according to gender (NIRAD study 6). J Clin Endocrinol Metab. 2012; 97:3759–3765.4. Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, Zhou Z. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf). 2011; 74:587–592.5. Koo BK, Chae S, Kim KM, Kang MJ, Kim EG, Kwak SH, Jung HS, Cho YM, Choi SH, Park YJ, Shin CH, Jang HC, Shin CS, Hwang D, Yi EC, Park KS. Identification of novel autoantibodies in type 1 diabetic patients using a high-density protein microarray. Diabetes. 2014; 63:3022–3032.6. Decochez K, Tits J, Coolens JL, Van Gaal L, Krzentowski G, Winnock F, Anckaert E, Weets I, Pipeleers DG, Gorus FK. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care. 2000; 23:838–844.7. Powell M, Prentice L, Asawa T, Kato R, Sawicka J, Tanaka H, Petersen V, Munkley A, Morgan S, Rees Smith B, Furmaniak J. Glutamic acid decarboxylase autoantibody assay using 125I-labelled recombinant GAD65 produced in yeast. Clin Chim Acta. 1996; 256:175–188.8. Torn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ. Participating Laboratories. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008; 51:846–852.9. Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, Songini M, Bonicchio S, Giorgino F, Bonifacio E, Bosi E, Buzzetti R. Non Insulin Requiring Autoimmune Diabetes (NIRAD) Study Group. Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care. 2010; 33:104–108.10. Yang L, Luo S, Huang G, Peng J, Li X, Yan X, Lin J, Wenzlau JM, Davidson HW, Hutton JC, Zhou Z. The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev. 2010; 26:579–584.11. DIAMOND Project Group. Incidence and trends of childhood type 1 diabetes worldwide 1990-1999. Diabet Med. 2006; 23:857–866.12. Kulmala P, Rahko J, Savola K, Vahasalo P, Sjoroos M, Reunanen A, Ilonen J, Knip M. Beta-cell autoimmunity, genetic susceptibility, and progression to type 1 diabetes in unaffected schoolchildren. Diabetes Care. 2001; 24:171–173.13. Samuelsson U, Sundkvist G, Borg H, Fernlund P, Ludvigsson J. Islet autoantibodies in the prediction of diabetes in school children. Diabetes Res Clin Pract. 2001; 51:51–57.14. Lundgren VM, Isomaa B, Lyssenko V, Laurila E, Korhonen P, Groop LC, Tuomi T. Botnia Study Group. GAD antibody positivity predicts type 2 diabetes in an adult population. Diabetes. 2010; 59:416–422.15. Zimmet PZ, Shaten BJ, Kuller LH, Rowley MJ, Knowles WJ, Mackay IR. Antibodies to glutamic acid decarboxylase and diabetes mellitus in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1994; 140:683–690.16. Barinas-Mitchell E, Kuller LH, Pietropaolo S, Zhang YJ, Henderson T, Pietropaolo M. The prevalence of the 65-kilodalton isoform of glutamic acid decarboxylase autoantibodies by glucose tolerance status in elderly patients from the cardiovascular health study. J Clin Endocrinol Metab. 2006; 91:2871–2877.17. Kordonouri O, Hartmann R, Gruters-Kieslich A, Knip M, Danne T. Age-specific levels of diabetes-related GAD and IA-2 antibodies in healthy children and adults. J Pediatr Endocrinol Metab. 2002; 15:47–52.18. Batstra MR, van Driel A, Petersen JS, van Donselaar CA, van Tol MJ, Bruining GJ, Grobbee DE, Dyrberg T, Aanstoot HJ. Glutamic acid decarboxylase antibodies in screening for autoimmune diabetes: influence of comorbidity, age, and sex on specificity and threshold values. Clin Chem. 1999; 45:2269–2272.19. Dabelea D, Ma Y, Knowler WC, Marcovina S, Saudek CD, Arakaki R, White NH, Kahn SE, Orchard TJ, Goldberg R, Palmer J, Hamman RF. Diabetes Prevention Program Research Group. Diabetes autoantibodies do not predict progression to diabetes in adults: the Diabetes Prevention Program. Diabet Med. 2014; 31:1064–1068.20. Ditzel HJ, Masaki Y, Nielsen H, Farnaes L, Burton DR. Cloning and expression of a novel human antibody-antigen pair associated with Felty's syndrome. Proc Natl Acad Sci U S A. 2000; 97:9234–9239.21. Orozco G, Eyre S, Hinks A, Bowes J, Morgan AW, Wilson AG, Wordsworth P, Steer S, Hocking L, Thomson W, Worthington J, Barton A. UKRAG consortium. Study of the common genetic background for rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2011; 70:463–468.22. Fransen K, Visschedijk MC, van Sommeren S, Fu JY, Franke L, Festen EA, Stokkers PC, van Bodegraven AA, Crusius JB, Hommes DW, Zanen P, de Jong DJ, Wijmenga C, van Diemen CC, Weersma RK. Analysis of SNPs with an effect on gene expression identifies UBE2L3 and BCL3 as potential new risk genes for Crohn's disease. Hum Mol Genet. 2010; 19:3482–3488.23. International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN). Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008; 40:204–210.24. Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 1998; 17:7151–7160.25. Knudsen SM, Frydenberg J, Clark BF, Leffers H. Tissue-dependent variation in the expression of elongation factor-1 alpha isoforms: isolation and characterisation of a cDNA encoding a novel variant of human elongation-factor 1 alpha. Eur J Biochem. 1993; 215:549–554.26. Chambers DM, Peters J, Abbott CM. The lethal mutation of the mouse wasted (wst) is a deletion that abolishes expression of a tissue-specific isoform of translation elongation factor 1alpha, encoded by the Eef1a2 gene. Proc Natl Acad Sci U S A. 1998; 95:4463–4468.27. Lee S, Francoeur AM, Liu S, Wang E. Tissue-specific expression in mammalian brain, heart, and muscle of S1, a member of the elongation factor-1 alpha gene family. J Biol Chem. 1992; 267:24064–24068.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Ubiquitin-Proteasome System and F-box Proteins in Pathogenic Fungi
- Purification and Identification of Ubiquitin Binding Proteins from Erythrocytes of Patients with Dementia
- Interaction between Brucella melitensis 16M and small ubiquitin-related modifier 1 and E2 conjugating enzyme 9 in mouse RAW264.7 macrophages
- Ubiquitin Ligases in Cholesterol Metabolism
- First Report and Characterization of Pestalotiopsis ellipsospora Causing Canker on Acanthopanax divaricatus