Yonsei Med J.
2013 Jul;54(4):839-844. 10.3349/ymj.2013.54.4.839.
Parenteral Nutrition Associated Cholestasis Is Earlier, More Prolonged and Severe in Small for Gestational Age Compared with Appropriate for Gestational Age Very Low Birth Weight Infants
- Affiliations
-
- 1Division of Neonatology, Department of Pediatrics, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea. Ranng@yuhs.ac
- 2Department of Pediatrics, Sung-Ae General Hospital, Seoul, Korea.
- KMID: 2158216
- DOI: http://doi.org/10.3349/ymj.2013.54.4.839
Abstract
- PURPOSE
We hypothesized that parenteral nutrition associated cholestasis (PNAC) would be more severe in small for gestational age (SGA) compared with appropriate for gestational age (AGA) very low birth weight (VLBW) infants.
MATERIALS AND METHODS
Sixty-one VLBW infants were diagnosed as PNAC with exposure to parenteral nutrition with elevation of direct bilirubin > or =2 mg/dL for > or =14 days. Twenty-one SGA infants and 40 AGA infants matched for gestation were compared.
RESULTS
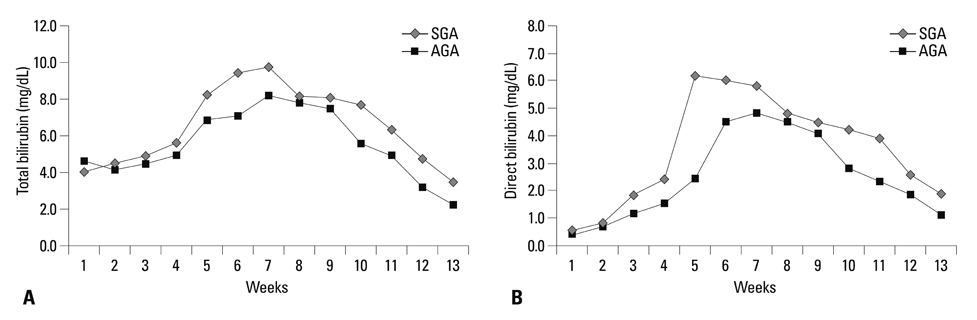
Compared with AGA infants, PNAC in SGA infants was diagnosed earlier (25+/-7 days vs. 35+/-14 days, p=0.002) and persisted longer (62+/-36 days vs. 46+/-27 days, p=0.048). Severe PNAC, defined as persistent elevation of direct bilirubin > or =4 mg/dL for more than 1 month with elevation of liver enzymes, was more frequent in SGA than in AGA infants (61% vs. 35%, p=0.018). The serum total bilirubin and direct bilirubin levels during the 13 weeks of life were significantly different in SGA compared with AGA infants. SGA infants had more frequent (76% vs. 50%, p=0.046), and persistent elevation of alanine aminotransferase.
CONCLUSION
The clinical course of PNAC is more persistent and severe in SGA infants. Careful monitoring and treatment are required for SGA infants.
Keyword
MeSH Terms
-
Bilirubin/blood
Case-Control Studies
Cholestasis/diagnosis/epidemiology/*etiology
Comorbidity
Female
Humans
Infant, Newborn
Infant, Premature, Diseases/epidemiology/etiology
*Infant, Small for Gestational Age
Infant, Very Low Birth Weight
Liver/metabolism/physiopathology
Male
Parenteral Nutrition/*adverse effects
Bilirubin
Figure
Reference
-
1. Beale EF, Nelson RM, Bucciarelli RL, Donnelly WH, Eitzman DV. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics. 1979; 64:342–347.
Article2. Shin JI, Namgung R, Park MS, Lee C. Could lipid infusion be a risk for parenteral nutrition-associated cholestasis in low birth weight neonates? Eur J Pediatr. 2008; 167:197–202.
Article3. Steinbach M, Clark RH, Kelleher AS, Flores C, White R, Chace DH, et al. Demographic and nutritional factors associated with prolonged cholestatic jaundice in the premature infant. J Perinatol. 2008; 28:129–135.
Article4. Merritt RJ. Cholestasis associated with total parenteral nutrition. J Pediatr Gastroenterol Nutr. 1986; 5:9–22.
Article5. Rosenberg A. The IUGR newborn. Semin Perinatol. 2008; 32:219–224.
Article6. Boehm G, Müller DM, Teichmann B, Krumbiegel P. Influence of intrauterine growth retardation on parameters of liver function in low birth weight infants. Eur J Pediatr. 1990; 149:396–398.
Article7. Baserga MC, Sola A. Intrauterine growth restriction impacts tolerance to total parenteral nutrition in extremely low birth weight infants. J Perinatol. 2004; 24:476–481.
Article8. Robinson DT, Ehrenkranz RA. Parenteral nutrition-associated cholestasis in small for gestational age infants. J Pediatr. 2008; 152:59–62.
Article9. Costa S, Maggio L, Sindico P, Cota F, De Carolis MP, Romagnoli C. Preterm small for gestational age infants are not at higher risk for parenteral nutrition-associated cholestasis. J Pediatr. 2010; 156:575–579.
Article10. Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966; 37:403–408.
Article11. Kubota A, Yonekura T, Hoki M, Oyanagi H, Kawahara H, Yagi M, et al. Total parenteral nutrition-associated intrahepatic cholestasis in infants: 25 years' experience. J Pediatr Surg. 2000; 35:1049–1051.
Article12. Btaiche IF, Khalidi N. Parenteral nutrition-associated liver complications in children. Pharmacotherapy. 2002; 22:188–211.
Article13. Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998; 27:131–137.
Article14. Carter BA, Shulman RJ. Mechanisms of disease: update on the molecular etiology and fundamentals of parenteral nutrition associated cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2007; 4:277–287.
Article15. Beath SV, Davies P, Papadopoulou A, Khan AR, Buick RG, Corkery JJ, et al. Parenteral nutrition-related cholestasis in postsurgical neonates: multivariate analysis of risk factors. J Pediatr Surg. 1996; 31:604–606.
Article16. Willis TC, Carter BA, Rogers SP, Hawthorne KM, Hicks PD, Abrams SA. High rates of mortality and morbidity occur in infants with parenteral nutrition-associated cholestasis. JPEN J Parenter Enteral Nutr. 2010; 34:32–37.
Article17. Bucuvalas JC, Goodrich AL, Blitzer BL, Suchy FJ. Amino acids are potent inhibitors of bile acid uptake by liver plasma membrane vesicles isolated from suckling rats. Pediatr Res. 1985; 19:1298–1304.
Article18. Li S, Nussbaum MS, Teague D, Gapen CL, Dayal R, Fischer JE. Increasing dextrose concentrations in total parenteral nutrition (TPN) causes alterations in hepatic morphology and plasma levels of insulin and glucagon in rats. J Surg Res. 1988; 44:639–648.
Article19. Aucott SW, Donohue PK, Northington FJ. Increased morbidity in severe early intrauterine growth restriction. J Perinatol. 2004; 24:435–440.
Article20. Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol. 2000; 182(1 Pt 1):198–206.
Article21. Regev RH, Lusky A, Dolfin T, Litmanovitz I, Arnon S, Reichman B. Israel Neonatal Network. Excess mortality and morbidity among small-for-gestational-age premature infants: a population-based study. J Pediatr. 2003; 143:186–191.
Article22. Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA. Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res. 1996; 39:390–394.
Article23. Lane RH, Tsirka AE, Gruetzmacher EM. Uteroplacental insufficiency alters cerebral mitochondrial gene expression and DNA in fetal and juvenile rats. Pediatr Res. 2000; 47:792–797.
Article24. Lane RH, Crawford SE, Flozak AS, Simmons RA. Localization and quantification of glucose transporters in liver of growth-retarded fetal and neonatal rats. Am J Physiol. 1999; 276(1 Pt 1):E135–E142.25. Cordano A. Clinical manifestations of nutritional copper deficiency in infants and children. Am J Clin Nutr. 1998; 67:5 Suppl. 1012S–1016S.
Article26. Peña MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr. 1999; 129:1251–1260.
Article27. Piper JM, Langer O, Xenakis EM, McFarland M, Elliott BD, Berkus MD. Perinatal outcome in growth-restricted fetuses: do hypertensive and normotensive pregnancies differ? Obstet Gynecol. 1996; 88:194–199.
Article28. Chen CY, Tsao PN, Chen HL, Chou HC, Hsieh WS, Chang MH. Ursodeoxycholic acid (UDCA) therapy in very-low-birth-weight infants with parenteral nutrition-associated cholestasis. J Pediatr. 2004; 145:317–321.
Article29. Koletzko B, Goulet O. Fish oil containing intravenous lipid emulsions in parenteral nutrition-associated cholestatic liver disease. Curr Opin Clin Nutr Metab Care. 2010; 13:321–326.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Total Parenteral Nutrition-associated Cholestasis in Very Low Birth Weight Infants
- The Risk Factors of Cholestasis in Very Low Birth Weight Infants
- Hepatobiliary Dysfunction in Very Low Birth Weight Infants Supported with Parenteral Nutrition
- The Effect of Early Enteral Trophic Feeding within 24 Hours after Birth in Extremely Low Birth Weight Infants of 26 Weeks and Less, and Birth Weight below 1,000 g
- Clinical Findings of Sepsis-Associated Cholestasis in the Neonates