Yonsei Med J.
2008 Oct;49(5):836-842. 10.3349/ymj.2008.49.5.836.
Granulocyte Stimulating Factor Attenuates Hypoxic-ischemic Brain Injury by Inhibiting Apoptosis in Neonatal Rats
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wonspark@skku.edu
- 2Department of Pediatrics, Hanil General Hospital, Seoul, Korea.
- 3Department of Pediatrics, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 4Department of Pediatrics, Samsung Biomedical Research Institute, Seoul, Korea.
- 5Department of Pediatrics, Buchon Sunchonhyang Hospital, Sunchonhyang University, Buchon, Korea.
- 6Department of Pediatrics, Young San Hospital, Chung-Ang University, Seoul, Korea.
- KMID: 2158203
- DOI: http://doi.org/10.3349/ymj.2008.49.5.836
Abstract
- PURPOSE
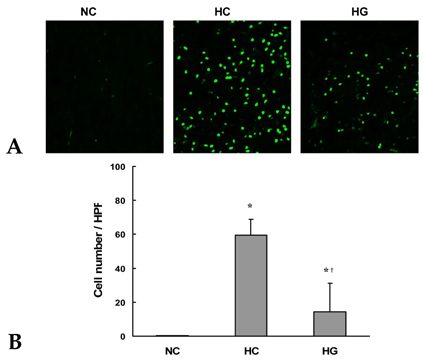
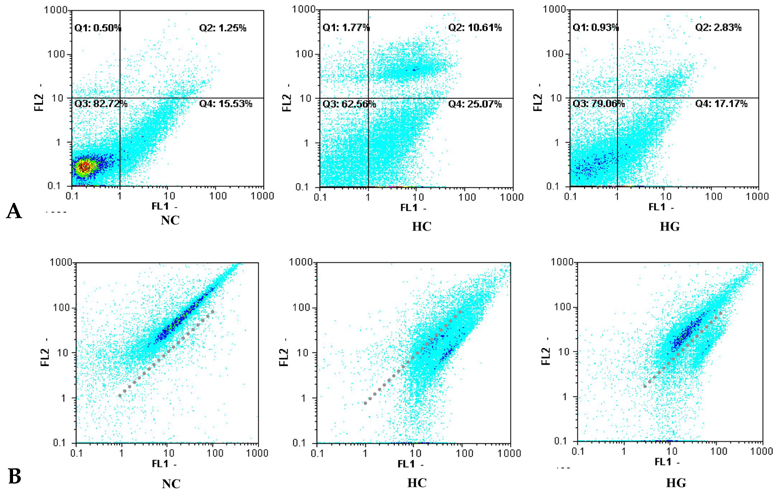
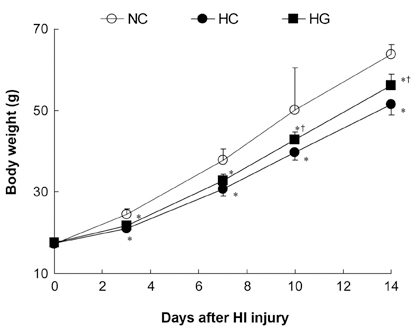
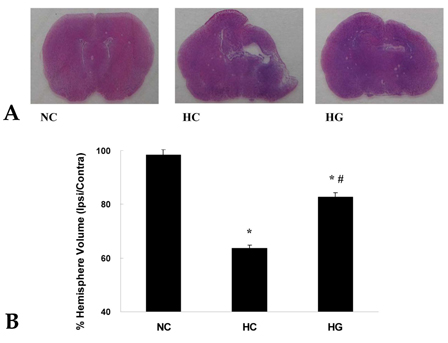
This study was undertaken to determine the neuroprotective effect of granulocyte stimulating factor (G-CSF) on neonatal hypoxic-ischemic brain injury. MATERIALS AND METHODS: Seven-day-old male newborn rat pups were subjected to 110 minutes of 8% oxygen following a unilateral carotid artery ligation. Apoptosis was identified by performing terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining and flow cytometry with a combination of fluorescinated annexin V and propidium iodide (PI) and JC-1 (5,5',6,6'-tetrachloro-1,1',3,3'-tetraethylbenzimidazolyl-carbocyanine iodide). The extent of cerebral infarction was evaluated at 2 weeks after recovery. RESULTS: With a single dose (50microgram/kg) of G-CSF treatment immediately after hypoxic-ischemic insult, hypoxia-ischemia induced increase in TUNEL-positive cells, annexinV+/PI- and JC-1 positive apoptotic cells in the ipsilateral cerebral cortex was significantly reduced at 24 hours, measured by flow cytometry, and the extent of cerebral infarction at 2 weeks after recovery was also significantly attenuated compared to the hypoxia-ischemia control group. CONCLUSION: Our data suggest that G-CSF is neuroprotective by inhibiting apoptosis, thereby reducing the ensuing cerebral infarction in a newborn rat pup model of cerebral hypoxia-ischemia (HI).
Keyword
MeSH Terms
-
Animals
Apoptosis/*drug effects
Brain/pathology
Cerebral Infarction/pathology/prevention & control
Flow Cytometry
Granulocyte Colony-Stimulating Factor/*pharmacology/therapeutic use
Hypoxia-Ischemia, Brain/*drug therapy/pathology
In Situ Nick-End Labeling
Male
Organ Size
Protective Agents/*pharmacology/therapeutic use
Rats
Rats, Sprague-Dawley
Weight Gain
Figure
Cited by 1 articles
-
Granulocyte Colony Stimulating Factor Attenuates Hyperoxia-Induced Lung Injury by Down-Modulating Inflammatory Responses in Neonatal Rats
Ga Won Jeon, Dong Kyung Sung, Yu Jin Jung, Soo Hyun Koo, Seo Heui Choi, Yun Sil Chang, Jong Beom Sin, Won Soon Park
Yonsei Med J. 2011;52(1):65-73. doi: 10.3349/ymj.2011.52.1.65.
Reference
-
1. Vannucci RC. Hypoxic-ischemic encephalopathy. Am J Perinatol. 2000. 17:113–120.
Article2. Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005. 25:431–439.
Article3. Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005. 64:763–769.
Article4. Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, et al. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005. 1058:120–128.
Article5. Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Schölzke MN, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003. 34:745–751.
Article6. Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005. 115:2083–2098.
Article7. Shyu WC, Lin SZ, Yang HI, Tzeng YS, Pang CY, Yen PS, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation. 2004. 110:1847–1854.
Article8. Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003. 458:327–328.9. Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006. 174:927–933.
Article10. Schabitz WR, Schneider A. Developing granulocyte-colony stimulating factor for the treatment of stroke: current status of clinical trials. Stroke. 2006. 37:1654.11. Solaroglu I, Cahill J, Jadhav V, Zhang JH. A novel neuroprotectant granulocyte-colony stimulating factor. Stroke. 2006. 37:1123–1128.12. Ahmad M, Fleit HB, Golightly MG, La Gamma EF. In vivo effect of recombinant human granulocyte colony-stimulating factor on phagocytic function and oxidative burst activity in septic neutropenic neonates. Biol Neonate. 2004. 86:48–54.
Article13. Carr R, Modi N. Haemopoietic growth factors for neonates: assessing risks and benefits. Acta Paediatr Suppl. 2004. 93:15–19.
Article14. Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007. 1145:227–238.
Article15. Keller M, Simbruner G, Górna A, Urbanek M, Tinhofer I, Griesmaier E, et al. Systemic application of granulocyte-colony stimulating factor and stem cell factor exacerbates excitotoxic brain injury in newborn mice. Pediatr Res. 2006. 59(4 Pt 1):549–553.
Article16. Park WS, Sung DK, Kang S, Koo SH, Kim YJ, Lee JH, et al. Neuroprotective effect of cycloheximide on hypoxic-ischemic brain injury in neonatal rats. J Korean Med Sci. 2006. 21:337–341.17. Park WS, Sung DK, Kang S, Koo SH, Kim YJ, Lee JH, et al. Therapeutic window for cycloheximide treatment after hypoxic-ischemic brain injury in neonatal rats. J Korean Med Sci. 2006. 21:490–494.
Article18. Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, et al. Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem. 2006. 96:1016–1027.19. Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic ischemic brain damage in the rat. Ann Neurol. 1981. 9:131–141.20. Vannucci RC, Towfighi J, Vannucci SJ. Secondary energy failure after cerebral hypoxia-ischemia in the immature rat. J Cereb Blood Flow Metab. 2004. 24:1090–1097.
Article21. Zuliani T, Duval R, Jayat C, Schnébert S, André P, Dumas M, et al. Sensitive and reliable JC-1 and TOTO-3 double staining to assess mitochondrial transmembrane potential and plasma membrane integrity: interest for cell death investigations. Cytometry A. 2003. 54:100–108.
Article22. Regeur L, Pakkenberg B. Optimizing sampling designs for volume measurements of components of human brain using a stereological method. J Microsc. 1989. 155:113–121.
Article23. Renvoizé C, Biola A, Pallardy M, Bréard J. Apoptosis: identification of dying cells. Cell Biol Toxicol. 1998. 14:111–120.24. Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995. 184:39–51.
Article25. Honda O, Kuroda M, Joja I, Asaumi J, Takeda Y, Akaki S, et al. Assessment of secondary necrosis of Jurkat cells using a new microscopic system and double staining method with annexin V and propidium iodide. Int J Oncol. 2000. 16:283–288.
Article26. Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, et al. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005. 12:162–176.
Article27. Linnik MD, Zobrist RH, Hatfield MD. Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke. 1993. 24:2002–2008. discussion 2008-9.
Article28. Johnson EM Jr, Deckwerth TL. Molecular mechanisms of developmental neuronal death. Annu Rev Neurosci. 1993. 16:31–46.
Article29. Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab. 1996. 16:195–201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neuroprotective Effect of Growth Hormone in Neonatal Rat with Hypoxic Ischemic Brain Injury
- Effect of the Heme Oxygenase Inhibitor on the Hypoxic Ischemic Brain Injury in the Neonatal Rat
- The Protective Effect of Ischemic and Hypoxic Preconditioning on Hypoxic-ischemic Brain Injury in the Neonatal Rat: 1H Magnetic Resonance Spectroscopic Study
- E-selectin Mrna and Protein Expression Increase Transiently After Unilateral Cerebral hypoxia-ischemia in Neonatal Rats
- Expression of ICAM-1 mRNA after Hypoxic-Ischemic Brain Injury in Neonatal Rats