Yonsei Med J.
2005 Feb;46(1):133-140. 10.3349/ymj.2005.46.1.133.
Cyclooxygenase-2 and p53 Expression as Prognostic Indicators in Conventional Renal Cell Carcinoma
- Affiliations
-
- 1Department of Urology, Ajou University School of Medicine, Suwon, Korea. sejoong@ajou.ac.kr
- 2Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- KMID: 2158123
- DOI: http://doi.org/10.3349/ymj.2005.46.1.133
Abstract
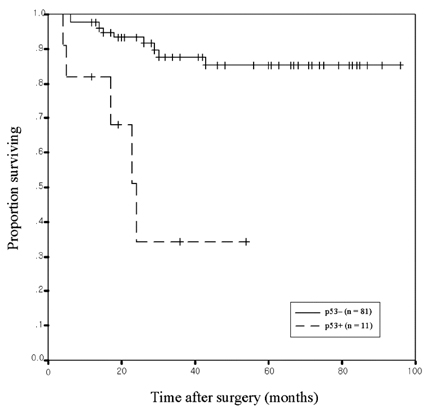
- The aim of this study was to investigate the relationship of cyclooxygenase (COX) -2 and p53 expression with prognosis in patients with conventional renal cell carcinoma (RCC). Formalin-fixed, paraffin-embedded tissue sections of conventional RCC from 92 patients, who had undergone radical nephrectomy, were examined for COX-2 and p53 expression by immunohistochemistry and compared with clinicopathological variables. The COX-2 expression significantly correlated only with tumor size (p=0.049), whereas the p53 expression profoundly correlated with the TNM stage (p=0.024), M stage (p=0.001), and metastasis (synchronous or metachronous; p= 0.004). The COX-2 overexpression did not significantly associate with p53 positivity (p=0.821). The survival rate of patients correlated with the p53 expression (p < 0.0001) but not with the COX-2 expression (p=0.7506). Multivariate analyses indicated that tumor size, M stage, and p53 expression were independent prognostic factors for cancer-specific survival. The COX-2 expression was not an independent factor. These results show that the increased expression of p53 was associated with metastasis and a worse prognosis in conventional RCC, which suggests that p53 might have played an important role in the progression of conventional RCC. The increased expression of COX-2 was associated only with tumor size, but may not be an important prognostic factor in conventional RCC. No association was observed between COX-2 overexpression and p53 positivity in conventional RCC.
Keyword
MeSH Terms
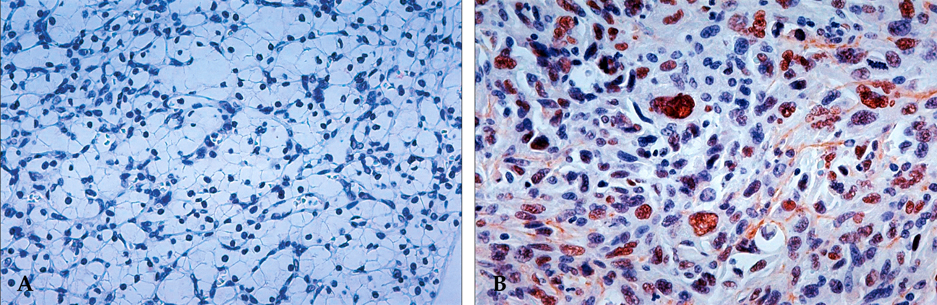
Figure
Reference
-
1. Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr. Rising incidence of renal cell cancer in the United States. JAMA. 1999. 281:1628–1631.2. Delahunt B. Histopathologic prognostic indicators for renal cell carcinoma. Semin Diagn Pathol. 1998. 15:68–76.3. Yasunaga Y, Shin M, Miki T, Okuyama A, Aozasa K. Prognostic factors of renal cell carcinoma: a multivariate analysis. J Surg Oncol. 1998. 68:11–18.4. Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998. 38:97–120.5. Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996. 1299:125–140.6. Masunaga R, Kohno H, Dhar DK, Ohno S, Shibakita M, Kinugasa S, et al. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin Cancer Res. 2000. 6:4064–4068.7. Joo YE, Rew JS, Seo YH, Choi SK, Kim YJ, Park CS, et al. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003. 37:28–33.8. Denkert C, Winzer KJ, Müller BM, Weichert W, Pest S, Köbel M, et al. Elevated expression of cyclooxgenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003. 97:2978–2987.9. Chen YJ, Wang LS, Wang PH, Lai CR, Yen MS, Ng HT, et al. High cyclooxygenase-2 expression in cervical adenocarcinomas. Gynecol Oncol. 2003. 88:379–385.10. Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001. 7:861–867.11. Yoshimura R, Sano H, Masuda C, Kawamura M, Tsubouchi Y, Chargui J, et al. Expression of cyclooxygenase-2 in prostate carcinoma. Cancer. 2000. 89:589–596.12. Shirahama T. Cyclooxygenase-2 expression is up-regulated in transitional cell carcinoma and its preneoplastic lesions in the human urinary bladder. Clin Cancer Res. 2000. 6:2424–2430.13. Cao Y, Prescott SM. Many actions of cyclooxygenase-2 in cellular dynamics and in cancer. J Cell Physiol. 2002. 190:279–286.14. Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999. 18:7908–7916.15. Pruthi RS, Derksen E, Gaston K. Cyclooxygenase-2 as a potential target in the prevention and treatment of genitourinary tumors: a review. J Urol. 2003. 169:2352–2359.16. Khan KN, Stanfield KM, Trajkovic D, Knapp DW. Expression of cyclooxygenase-2 in canine renal cell carcinoma. Vet Pathol. 2001. 38:116–119.17. Okamoto T, Hara A, Hino O. Down-regulation of cyclooxygenase-2 expression but up-regulation of cyclooxygenase-1 in renal carcinomas of the Eker (TSC2 gene mutant) rat model. Cancer Sci. 2003. 94:22–25.18. Miyata Y, Koga S, Kanda S, Nishikido M, Hayashi T, Kanetake H. Expression of cyclooxygenase-2 in renal cell carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, expression of matrix metalloproteinase-2, and survival. Clin Cancer Res. 2003. 9:1741–1749.19. Yoshimura R, Matsuyama M, Kawahito Y, Tsuchida K, Kuratsukuri K, Takemoto Y, et al. Study of cyclooxygenase-2 in renal cell carcinoma. Int J Mol Med. 2004. 13:229–233.20. Girgin C, Tarhan H, Hekimgil M, Sezer A, Gürel G. p53 mutations and other prognostic factors of renal cell carcinoma. Urol Int. 2001. 66:78–83.21. Uchida T, Gao JP, Wang C, Jiang SX, Muramoto M, Satoh T, et al. Clinical significance of p53, mdm2, and bcl-2 proteins in renal cell carcinoma. Urology. 2002. 59:615–620.22. Papadopoulos I, Rudolph P, Weichert-Jacobsen K. Value of p53 expression, cellular proliferation, and DNA content as prognostic indicators in renal cell carcinoma. Eur Urol. 1997. 32:110–117.23. Hofmockel G, Wittmann A, Dammrich J, Bassukas ID. Expression of p53 and bcl-2 in primary locally confined renal cell carcinomas: no evidence for prognostic significance. Anticancer Res. 1996. 16:3807–3811.24. Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, et al. TNM staging of renal cell carcinoma: Workgroup No. 3. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997. 80:992–993.25. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982. 6:655–663.26. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–241.27. Hara S, Kondo Y, Matsuzawa I, Hashimoto Y, Kimura G, Akimoto M, et al. Expression of cyclooxygenase-2 in human bladder and renal cell carcinoma. Adv Exp Med Biol. 2002. 507:123–126.28. Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998. 58:362–366.29. Chan TA, Morin PJ, Vogelstein B, Kinzler KW. Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci USA. 1998. 95:681–686.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Expression of p53 Oncogene Products, PCNA Index and DNA Ploidy in Renal Cell Carcinoma
- Prognostic Implication of p53 Immunohistochemical Staining in Patients with Localized Renal Cell Carcinoma after Radical Nephrectomy
- Comparison of Rb and p53 Protein Expression with Stage and Grade as a Prognostic Value in Renal Cell Carcinoma
- Comparative Study on p53 Expression and Clinical Charateristics in Renal Cell Carcinoma
- The Study of p53 Expression and DNA Ploidy in Colorectal Carcinoma