J Korean Med Sci.
2013 Jun;28(6):840-847. 10.3346/jkms.2013.28.6.840.
Genome-Wide Association Study of Lung Cancer in Korean Non-Smoking Women
- Affiliations
-
- 1Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea. ychong1@snu.ac.kr
- 2Institute of Environmental Medicine, Seoul National University Medical Research Center, Seoul, Korea.
- 3Department of Epidemiology and Biostatistics, Seoul National University School of Public Health, Seoul, Korea.
- 4Department of Medical Humanities and Social Sciences, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 5Integrated Research Center for Genome Polymorphism, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 6Department of Biochemistry, School of Medicine, Kyungpook National University, Daegu, Korea.
- 7Genomic Research Center for Lung and Breast/Ovarian Cancers, Korea University Anam Hospital, Seoul, Korea.
- 8Division of Oncology/Hematology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea.
- 9Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul, Korea.
- 10Department of Thoracic and Cardiovascular Surgery, Seoul National University College of Medicine, Seoul, Korea.
- 11Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea.
- 12Division of Pulmonary and Critical Care, Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 13Department of Microbiology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 14Department of Thoracic and Cardiovascular Surgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2158030
- DOI: http://doi.org/10.3346/jkms.2013.28.6.840
Abstract
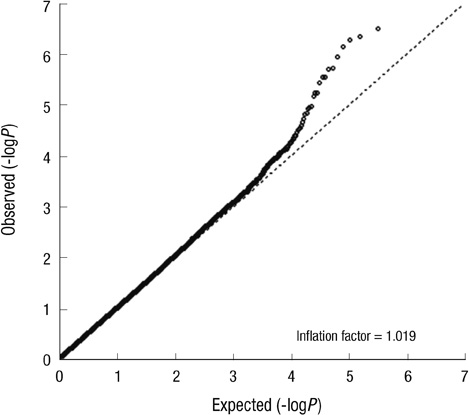
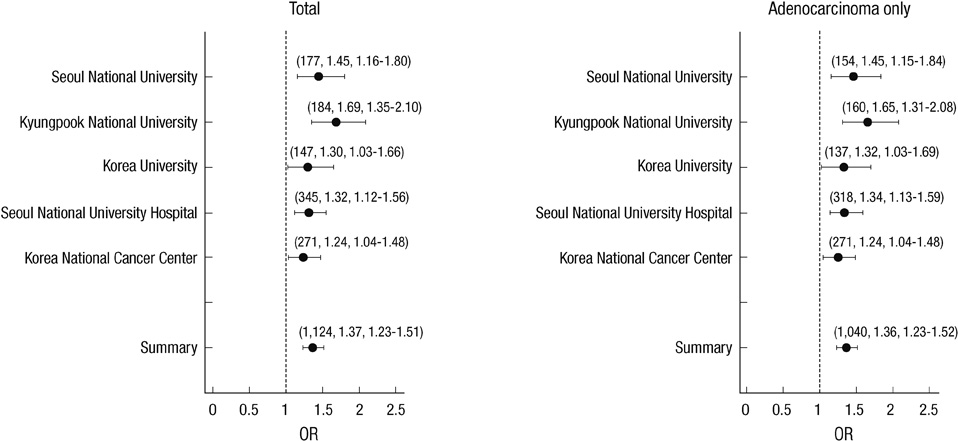
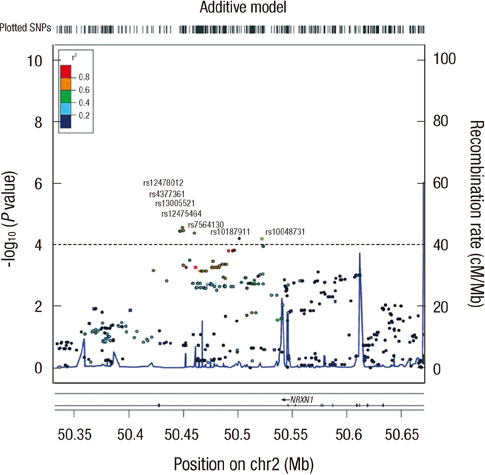
- Lung cancer in never-smokers ranks as the seventh most common cause of cancer death worldwide, and the incidence of lung cancer in non-smoking Korean women appears to be steadily increasing. To identify the effect of genetic polymorphisms on lung cancer risk in non-smoking Korean women, we conducted a genome-wide association study of Korean female non-smokers with lung cancer. We analyzed 440,794 genotype data of 285 cases and 1,455 controls, and nineteen SNPs were associated with lung cancer development (P < 0.001). For external validation, nineteen SNPs were replicated in another sample set composed of 293 cases and 495 controls, and only rs10187911 on 2p16.3 was significantly associated with lung cancer development (dominant model, OR of TG or GG, 1.58, P = 0.025). We confirmed this SNP again in another replication set composed of 546 cases and 744 controls (recessive model, OR of GG, 1.32, P = 0.027). OR and P value in combined set were 1.37 and < 0.001 in additive model, 1.51 and < 0.001 in dominant model, and 1.54 and < 0.001 in recessive model. The effect of this SNP was found to be consistent only in adenocarcinoma patients (1.36 and < 0.001 in additive model, 1.49 and < 0.001 in dominant model, and 1.54 and < 0.001 in recessive model). Furthermore, after imputation with HapMap data, we found regional significance near rs10187911, and five SNPs showed P value less than that of rs10187911 (rs12478012, rs4377361, rs13005521, rs12475464, and rs7564130). Therefore, we concluded that a region on chromosome 2 is significantly associated with lung cancer risk in Korean non-smoking women.
MeSH Terms
-
Adenocarcinoma/*genetics/pathology
Adult
Aged
Asian Continental Ancestry Group/*genetics
Cell Adhesion Molecules, Neuronal/*genetics
Chromosomes, Human, Pair 2
Female
*Genome-Wide Association Study
Genotype
Humans
Logistic Models
Lung Neoplasms/*genetics/pathology
Models, Genetic
Nerve Tissue Proteins/*genetics
Odds Ratio
Polymorphism, Single Nucleotide
Republic of Korea
Cell Adhesion Molecules, Neuronal
Nerve Tissue Proteins
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.2. Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers: a different disease. Nat Rev Cancer. 2007; 7:778–790.3. Torok S, Hegedus B, Laszlo V, Hoda MA, Ghanim B, Berger W, Klepetko W, Dome B, Ostoros G. Lung cancer in never smokers. Future Oncol. 2011; 7:1195–1211.4. Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst. 2008; 100:1552–1556.5. Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008; 40:616–622.6. Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008; 452:633–637.7. McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008; 40:1404–1406.8. Rafnar T, Sulem P, Stacey SN, Geller F, Gudmundsson J, Sigurdsson A, Jakobsdottir M, Helgadottir H, Thorlacius S, Aben KK, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009; 41:221–227.9. Truong T, Hung RJ, Amos CI, Wu X, Bickeböller H, Rosenberger A, Sauter W, Illig T, Wichmann HE, Risch A, et al. Replication of lung cancer susceptibility loci at chromosomes 15q25, 5p15, and 6p21: a pooled analysis from the International Lung Cancer Consortium. J Natl Cancer Inst. 2010; 102:959–971.10. Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager M, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009; 85:679–691.11. Wang Y, Broderick P, Webb E, Wu X, Vijayakrishnan J, Matakidou A, Qureshi M, Dong Q, Gu X, Chen WV, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008; 40:1407–1409.12. Rafnar T, Sulem P, Besenbacher S, Gudbjartsson DF, Zanon C, Gudmundsson J, Stacey SN, Kostic JP, Thorgeirsson TE, Thorleifsson G, et al. Genome-wide significant association between a sequence variant at 15q15.2 and lung cancer risk. Cancer Res. 2011; 71:1356–1361.13. Kohno T, Kunitoh H, Shimada Y, Shiraishi K, Ishii Y, Goto K, Ohe Y, Nishiwaki Y, Kuchiba A, Yamamoto S, et al. Individuals susceptible to lung adenocarcinoma defined by combined HLA-DQA1 and TERT genotypes. Carcinogenesis. 2010; 31:834–841.14. Pande M, Spitz MR, Wu X, Gorlov IP, Chen WV, Amos CI. Novel genetic variants in the chromosome 5p15.33 region associate with lung cancer risk. Carcinogenesis. 2011; 32:1493–1499.15. Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps DL, Qin S, Minx P, Wilson RK, Hood L, et al. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002; 79:587–597.16. Ching MS, Shen Y, Tan WH, Jeste SS, Morrow EM, Chen X, Mukaddes NM, Yoo SY, Hanson E, Hundley R, et al. Deletions of NRXN1 (neurexin-1) predispose to a wide spectrum of developmental disorders. Am J Med Genet B Neuropsychiatr Genet. 2010; 153B:937–947.17. Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009; 459:569–573.18. Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, Sonnenblick LI, Alvarez Retuerto AI, Imielinski M, Hadley D, Bradfield JP, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009; 5:e1000536.19. Pedrosa E, Kaushik S, Lachman HM. ChIP-chip analysis of neurexins and other candidate genes for addiction and neuropsychiatric disorders. J Neurogenet. 2010; 24:5–17.20. Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, Li MD. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Hum Mol Genet. 2008; 17:1569–1577.21. Hsiung CA, Lan Q, Hong YC, Chen CJ, Hosgood HD, Chang IS, Chatterjee N, Brennan P, Wu C, Zheng W, et al. The 5p15.33 locus is associated with risk of lung adenocarcinoma in never-smoking females in Asia. PLoS Genet. 2010; 6:e1001051.22. Li Y, Sheu CC, Ye Y, de Andrade M, Wang L, Chang SC, Aubry MC, Aakre JA, Allen MS, Chen F, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010; 11:321–330.23. Ahn MJ, Won HH, Lee J, Lee ST, Sun JM, Park YH, Ahn JS, Kwon OJ, Kim H, Shim YM, et al. The 18p11.22 locus is associated with never smoker non-small cell lung cancer susceptibility in Korean populations. Hum Genet. 2012; 131:365–372.24. Hu Z, Wu C, Shi Y, Guo H, Zhao X, Yin Z, Yang L, Dai J, Hu L, Tan W, et al. A genome-wide association study identifies two new lung cancer susceptibility loci at 13q12.12 and 22q12.2 in Han Chinese. Nat Genet. 2011; 43:792–796.25. Lan Q, Hsiung CA, Matsuo K, Hong YC, Seow A, Wang Z, Hosgood HD 3rd, Chen K, Wang JC, Chatterjee N, et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never smoking women in Asia. Nat Genet. 2012; 44:1330–1335.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Short History of the Genome-Wide Association Study: Where We Were and Where We Are Going
- Passive Smoking and Lung Cancer
- Association between Lung Cancer Susceptibility Variants Identified by Genome-Wide Association Studies and the Survival of Non-Small Cell Lung Cancer
- The Role of the Epidemiological Causality of the Association between Smoking and Lung Cancer
- The association between smoking and asthma