J Korean Med Sci.
2006 Aug;21(4):728-732. 10.3346/jkms.2006.21.4.728.
Vigabatrin and Visual Field Defects in Pediatric Epilepsy Patients
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea. tsko@amc.seoul.kr
- 2Department of Ophthalmology, College of Medicine, University of Ulsan, Asan Medical Center, Seoul, Korea.
- KMID: 2157829
- DOI: http://doi.org/10.3346/jkms.2006.21.4.728
Abstract
- We studied the prevalence, type and severity of vigabatrin (VGB)-attributed visual field defects (VFDs), and used these data to assess the associated risk factors in pediatric patients. Medical records were retrospectively reviewed for 67 pediatric patients who received VGB alone or in combination with other antiepileptic drugs, and who had undergone visual field examinations using a Humphrey visual field analyzer. Of the 67 patients, 15 had VGB-attributed VFDs: 13 had nasal arcuate type, 1 had nasal and temporal constricted type and 1 had nasal constricted type. In terms of severity, 7 patients had Grade I VGB-attributed VFDs, 5 had Grade II, 2 had Grade III, and 1 had Grade IV. Although there were no significant differences between the VFD and non-VFD groups with regards to all tested parameters, there were no cases of VGB-attributed VFDs in patients with total treatment durations <2 yr and cumulative doses <10 g/kg. In conclusion, the prevalence of VGB-attributed VFDs in VGB-treated pediatric epilepsy patients was 22%. The high frequency of VGB-attributed VFDs indicates that physicians should inform all patients of this risk prior to VGB treatment and perform periodic visual field examinations.
Keyword
MeSH Terms
-
Visual Fields/drug effects
Vision Disorders/*chemically induced
Vigabatrin/adverse effects/*therapeutic use
Time Factors
Risk Factors
Retrospective Studies
Male
Humans
Female
Epilepsy/*drug therapy
Drug Therapy, Combination
Drug Monitoring/statistics & numerical data
Child
Anticonvulsants/adverse effects/*therapeutic use
Adult
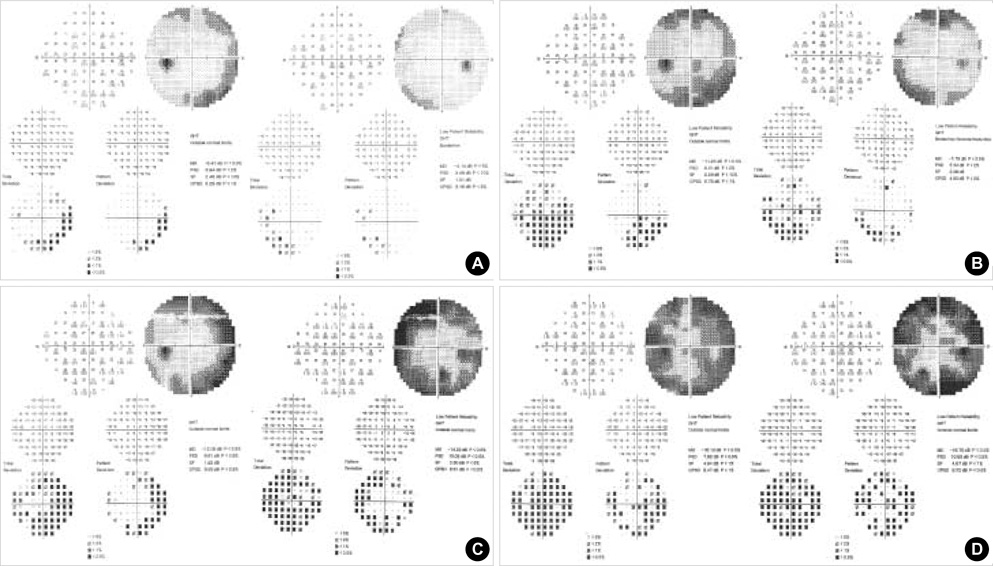
Figure
Reference
-
1. Ben-Menachem E. Vigabatrin. Epilepsia. 1995. 36:Suppl 2. 95–104.
Article2. Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999. 14:71–74.3. Eke T, Talbot JF, Lawden MC. Severe persistent visual field constriction associated with vigabatrin. BMJ. 1997. 314:180–181.
Article4. Hardus P, Verduin WM, Postma G, Stilma JS, Berendschot TT, van Veelen CW. Concentric contraction of the visual field in patients with temporal lobe epilepsy and its association with the use of vigabatrin medication. Epilepsia. 2000. 41:581–587.
Article5. Hardus P, Verduin WM, Postma G, Stilma JS, Berendschot TT, van Veelen CW. Long term changes in the visual fields of patients with temporal lobe epilepsy using vigabatrin. Br J Ophthalmol. 2000. 84:788–790.6. Manuchehri K, Goodman S, Siviter L, Nightingale S. A controlled study of vigabatrin and visual abnormalities. Br J Ophthalmol. 2000. 84:499–505.
Article7. Vanhatalo S, Nousiainen I, Eriksson K, Rantala H, Vainionpää L, Mustonen K, Äärimaa T, Alen R, Aine MR, Byring R, Hirvasniemi A, Nuutila A, Walden T, Ritanen-Mohammed UM, Karttunen-Lewandowski P, Pohjola LM, Kaksonen S, Jurvelin P, Granstromet LM. Visual field constriction in 91 Finnish children treated with vigabatrin. Epilepsia. 2000. 43:748–756.8. Malmgren K, Ben-Menachen E, Frisén L. Vigabatrin visual toxicity: Evolution and dose dependence. Epilepsia. 2001. 42:609–615.
Article9. Kälviäinen R, Nousiainen I, Mäntyjärvi M, Nikoskelainen E, Partanen J, Partanen K, Riekkinen P. Vigabatrin, a GABAergic antiepileptic drug, causes concentric visual field defects. Neurology. 1999. 53:922–926.
Article10. Wild JM, Martinez C, Reinshagen G, Harding GF. Characteristics of a unique visual field defect attributed to vigabatrin. Epilepsia. 1999. 40:1784–1794.
Article11. Krauss GL, Johnson MA, Miller NR. Vigabatrin-associated retinal cone system dysfunction: electroretinogram and ophthalmologic findings. Neurology. 1998. 50:614–618.
Article12. Arndt CF, Derambure P, Defoort-Dhellemmes S, Hache JC. Outer retinal dysfunction in patients treated with vigabatrin. Neurology. 1999. 52:1201–1205.
Article13. Iannetti P, Spalice A, Perla FM, Conicella E, Raucci U, Bizzarri B. Visual field constriction in children with epilepsy on vigabatrin treatment. Pediatrics. 2000. 106:838–842.
Article14. Vanhatalo S, Pääkkönen L, Nousiainen I. Visual field constriction in children treated with vigabatrin. Neurology. 1999. 52:1713–1714.
Article15. Gross-Tsur V, Banin E, Shahar E, Shalev RS, Lahat E. Visual impairment in children with epilepsy treated with vigabatrin. Ann Neurol. 2000. 48:60–64.
Article16. Wohlrab G, Boltshauser E, Schmitt B, Schriever S, Landau K. Visual field constriction is not limited to children treated with vigabatrin. Neuropediatrics. 1999. 30:130–132.
Article17. Miller NR, Johnson MA, Paul SR, Girkin CA, Perry JD, Endres M, Krauss GL. Visual dysfunction receiving vigabatrin: Clinical and electrophysiologic findings. Neurology. 1999. 53:2082–2087.18. Toggweiler S, Wieser HG. Concenteric visual filed restriction under vigabatrin therapy: extent depends on the duration of drug intake. Seizure. 2001. 10:420–423.19. Appleton RE, Peters AC, Mumford JP, Shaw DE. Randomized, placebo-controlled study of vigabatrin as first-line treatment of infantile spasms. Epilepsia. 1999. 40:1627–1633.20. Schmit T, Ruther K, Jokiel B, Pfeiffer S, Tiel-Wilck K, Schmitz B. Is visual field constriction in epilepsy patients treated with vigabatrin reversible? J Neurol. 2002. 249:1066–1071.21. Johnson MA, Krauss GL, Miller NR, Medura M, Paul SR. Visual function loss from vigabatrin: effect of stopping the drug. Neurology. 2000. 55:40–45.
Article22. Krakow K, Polizzi G, Riordan-Eva P, Holder G, MacLeod WN, Fish DR. Recovery of visual constriction following discontinuation of vigabatrin. Seizure. 2000. 9:287–290.23. Fledelius HC. Vigabatrin associated visual field constriction in a longitudinal series. Reversibility suggested after drug withdrawal. Acta Ophthalmol Scand. 2003. 81:41–46.24. Versino M, Veggiotti P. Reversibility of vigabatrin-induced visualfield defect. Lancet. 1999. 354:486.
Article25. Giordano L, Valseriati D, Vignoli A, Morescalchi F, Gandolfo E. Another case of reversibility of visual-defect induced by vigabatrin monotherapy: is young age a favorable factor? Neurol Sci. 2000. 21:185–186.26. Nousiainen I, Kalviainen R, Mantyjarvi M. Contrast and glare sensitivity in epilepsy patients treated with vigabatrin or carbamazepine monotherapy compared with healthy volunteers. Br J Ophthalmol. 2000. 84:622–625.
Article27. Lawden MC, Eke T, Degg C, Harding GF, Wild JM. Visual field defects associated with vigabatrin therapy. J Neurol Neurosurg Psychiatry. 1999. 67:716–722.
Article28. Daneshvar H, Racette L, Coupland SG, Kertes PJ, Guberman A, Zackon D. Symptomatic and asymptomatic visual loss in patients taking vigabatrin. Ophthalmology. 1999. 106:1792–1798.
Article29. Hardus P, Verduin WM, Engelsman M, Edelbroek PM, Segers JP, Berendschot TT, Stilma JS. Visual field loss associated with vigabatrin: quantification and relation to dosage. Epilepsia. 2001. 42:262–267.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Visual Field Defect in the Vigabatrin-treated Pediatric Patients
- Visual Field Changes in Children with Vigabatrin Treatment
- Assessment of the Relationship between Vigabatrin and Visual Field Defect in Children
- Effect of vigabatrin in pediatric intractable epilepsy
- Visual Field Changes in Pediatric and Adolescent Patients Treated with Vigabatrin