J Korean Med Sci.
2005 Dec;20(6):1000-1005. 10.3346/jkms.2005.20.6.1000.
Investigation of Early Protein Changes in the Urinary Bladder Following Partial Bladder Outlet Obstruction by Proteomic Approach
- Affiliations
-
- 1The Proteomics Research Group, Department of Urology, Dankook University College of Medicine, Cheonan, Korea. killtumor@dankook.ac.kr
- 2Department of Anesthiology, Dankook University College of Medicine, Cheonan, Korea.
- 3Department of Ophthalmology, Dankook University College of Medicine, Cheonan, Korea.
- 4Department of Bio-Resources Science, Dankook University, Cheonan, Korea.
- 5Department of Surgery, Choongmu Hospital, Cheonan, Korea.
- KMID: 2157752
- DOI: http://doi.org/10.3346/jkms.2005.20.6.1000
Abstract
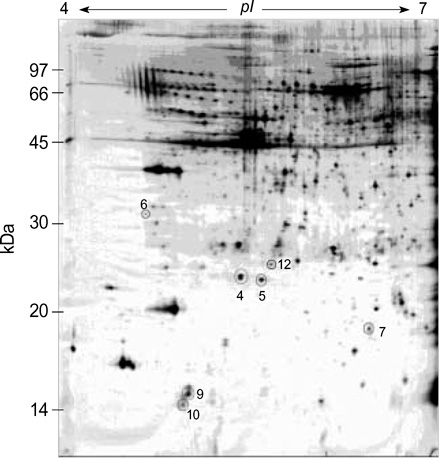
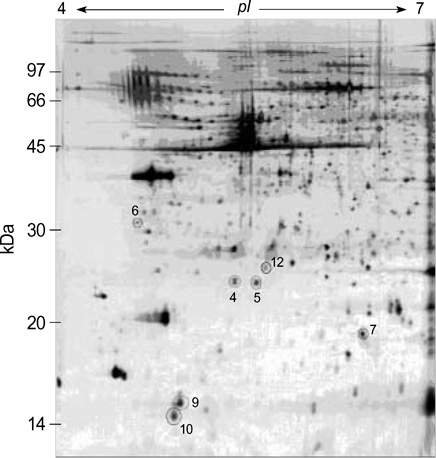
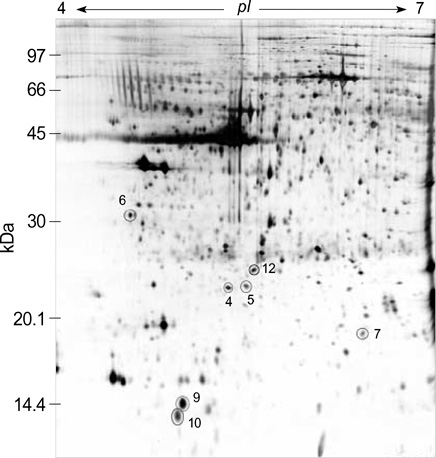
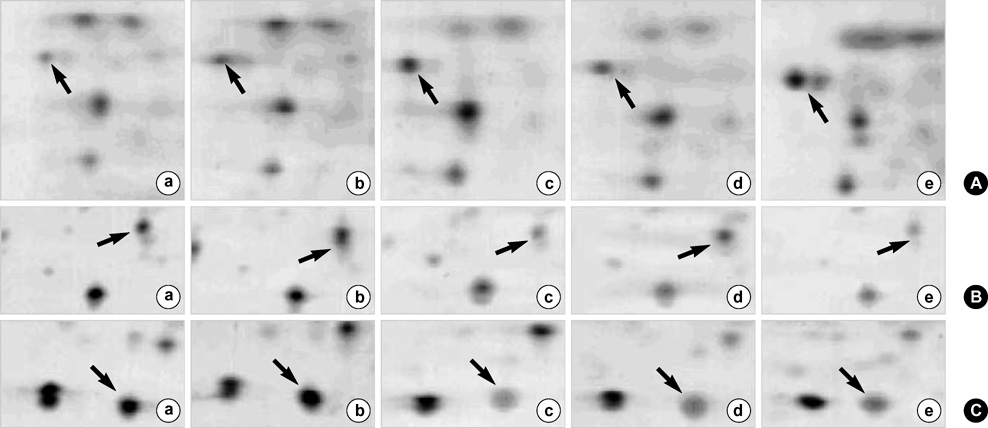
- We investigated the pathophysiological mechanism by proteomic approach as a possible tool to detect the marker proteins to develop lower urinary tract symptoms following bladder outlet obstruction (BOO). Rats were randomized into 3 groups; control, sham operation and BOO groups. BOO group was divided into 1, 3, and 5 day-group. Conventional proteomics was performed with high resolution 2-D gel electrophoresis followed by computational image analysis and protein identification using mass spectrometry using rat urinary bladders. A comparison of bladder of BOO group with control bladder showed that three proteins of optineurin, thioredoxin and preprohaptoglobin were over-expressed in the bladder of BOO group. In addition, four proteins, such as peroxiredoxin 2, transgelin, hippocampal cholinergic neurostimulating peptide (HCNP) and beta-galactoside-binding lectin, were under-expressed in the bladder of BOO group. These data supported that downregulation of HCNP might make detrusor muscle be supersensitive to acetylcholine, up-regulation of optineurin means the protection of nerve injury, and down-regulation of transgelin means the decreased contractility of detrusor muscle. Beside these proteins, other proteins are related to oxidative stress or have a nonspecific function in this study. However more information is needed in human bladder tissue for clinical usage.
MeSH Terms
-
Animals
Bladder/*metabolism
Bladder Neck Obstruction/genetics/*metabolism
Down-Regulation
Electrophoresis, Gel, Two-Dimensional
Female
Gene Expression
Proteins/genetics/isolation and purification/*metabolism
Proteomics
Rats
Rats, Sprague-Dawley
Research Support, Non-U.S. Gov't
Spectrometry, Mass, Matrix-Assisted Laser Desorption-Ionization
Up-Regulation
Figure
Reference
-
1. Caine M. The present role of alpha-adrenergic blockers in the treatment of benign prostatic hypertrophy. J Urol. 1986. 136:1–4.
Article2. Abrams PH, Farrar DJ, Turner-Warwick RT, Whiteside CG, Feneley RC. The results of prostatectomy: a symptomatic and urodynamic analysis of 152 patients. J Urol. 1979. 121:640–642.
Article3. Barry MJ, Meigs JB. Lepor H, editor. The natural history of benign prostatic hyperplasia. Prostatic disease. 2000. Philadelphia: Saunders;106–115.4. Anderson KE. Storage and voiding symptoms: pathophysiologic aspects. Urology. 2003. 62:3–10.
Article5. Levin RM, Chichester P, Hass MA, Goslin JA, Buttyan R. Obstructive bladder dysfunction: morphological, biochemical and molecular changes. Eur Urol suppl. 2002. 1:14–20.
Article6. Santarosa R, Colombel MC, Kaplan S, Monson F, Levin RM, Buttyan R. Hyperplasia and apoptosis. Opposing cellular processes that regulate the response of the rabbit bladder to transient outlet obstruction. Lab Invest. 1994. 70:503–510.7. Chen MW, Krasnapolsky L, Levin RM, Buttyan R. An early molecular response induced by acute overdistension of the rabbit urinary bladder. Mol Cell Biochem. 1994. 132:39–44.
Article8. Flynn BJ, Mian HS, Cera PJ, Kabler RL, Mowad JJ, Cavanaugh AH, Rothblum LI. Early molecular changes in bladder hypertrophy due to bladder outlet obstruction. Urology. 2002. 59:978–982.
Article9. Steers WD. Overactive bladder (OAB): What we thought we knew and what we know today. Eur Urol suppl. 2002. 1:3–10.
Article10. Levin RM, Wein AJ, Buttyan R, Monson FC, Longhurst PA. Update on bladder smooth-muscle physiology. World J Urol. 1994. 12:226–232.
Article11. Levin RM, Brading AF, Mills IW, Longhust PA. Lepor H, editor. Experimental models of bladder obstruction. Prostatic disease. 2000. Philadelphia: Saunders;169–196.12. Wilkins MR, Sanchez JC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, Williams KL. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996. 13:19–50.
Article13. Pandey A, Mann M. Proteomics to study genes and genomes. Nature. 2000. 405:837–846.
Article14. Adam GC, Cravatt BF, Sorensen EJ. Profiling the specific reactivity of the proteome with non-directed activity-based probes. Chem Biol. 2001. 8:81–95.
Article15. Chambers G, Lawrie L, Cash P, Murray GI. Proteomics: a new approach to the study of disease. J Pathol. 2000. 192:280–288.
Article16. Katada E, Mitake S, Matsukawa N, Otsuka Y, Tsugu Y, Fujimori O, Ojika K. Distribution of hippocampal cholinergic neurostimulating peptide (HCNP)-like immunoreactivity in organs and tissues of young Wistar rats. Histochem Cell Biol. 1996. 105:43–51.
Article17. Katada E, Ojika K, Mitake S, Ueda R. Neuronal distribution and subcellular localization of HCNP-like immunoreactivity in rat small intestine. J Neurocytol. 2000. 29:199–207.18. Ojika K, Ueki Y, Mitake S, Tsugu Y, Otsuka Y, Katada E. Demonstration of the biological activity of peptide fragments related to human and rat hippocampal cholinergic neurostimulating peptide (HCNP). Neurosci Lett. 1996. 215:127–130.
Article19. Sarfarazi M, Rezaie T. Optineurin in primary open angle glaucoma. Ophthalmol Clin North Am. 2003. 16:529–541.
Article20. Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002. 295:1077–1079.
Article21. Shapland C, Hsuan JJ, Totty NF, Lawson D. Purification and properties of transgelin: a transformation and shape change sensitive actin-gelling protein. J Cell Biol. 1993. 121:1065–1073.
Article22. Lawson D, Harrison M, Shapland C. Fibroblast transgelin and smooth muscle SM22 alpha are the same protein, the expression of which is down-regulated in many cell lines. Cell Motil Cytoskeleton. 1997. 38:250–257.23. Zeidan A, Sward K, Nordstrom I, Ekblad E, Zhang JC, Parmacek MS, Hellstrand P. Ablation of SM22alpha decreases contractility and actin contents of mouse vascular smooth muscle. FEBS Lett. 2004. 562:141–146.24. Stefankova P, Kollarova M, Barak I. Thioredoxin-structural and functional complexity. Gen Physiol Biophys. 2005. 24:3–11.25. Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxid Redox Signal. 2005. 7:823–828.
Article26. Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM, Baumman H, Halliwell B, Lim SK. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 2000. 58:1033–1044.
Article27. Dietz KJ, Horling F, Konig J, Baier M. The function of the chloroplast 2-cysteine peroxiredoxin in peroxide detoxification and its regulation. J Exp Bot. 2002. 53:1321–1329.
Article28. Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR Jr. Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. Am J Pathol. 1991. 147:1016–1028.29. Imbe H, Okamoto K, Kadoya T, Horie H, Senba E. Galectin-1 is involved in the potentiation of neuropathic pain in the dorsal horn. Brain Res. 2003. 993:72–83.
Article30. Stillman BN, Mischel PS, Baum LG. New roles for galectins in brain tumors--from prognostic markers to therapeutic targets. Brain Pathol. 2005. 15:124–132.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Investigation of Oxidative Stress and Development of Lower Urinary Tract Symptoms in the Urinary Bladder following Partial Bladder Outlet Obstruction by Proteomic Approach
- The Role of Peripheral and Spinal alpha1-adrenoceptor in Bladder Overactivity Induced by Partial Bladder Outlet Obstruction in Rat
- Effect of Bladder Outlet Obstruction on Blood Flow and Tissue Collagen in Rat Bladder
- Changes in Detrusor and Urinary Growth Factors According to the Bladder Function after Partial Bladder Outlet Obstruction in Rat
- Female Acute Urinary Retention Caused by Urethral Diverticulum