Korean J Neurotrauma.
2014 Apr;10(1):1-5. 10.13004/kjnt.2014.10.1.1.
Changes of the Electrophysiological Study in Dogs with Acute Spinal Cord Injury
- Affiliations
-
- 1Laboratory of Stem Cell Therapy, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. neuri71@gmail.com
- 2Department of Rehabilitation Medicine, Hallym University Medical Center, Seoul, Korea.
- 3Department of Neurosurgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Rehabilitation Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Neurosurgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea.
- KMID: 2156089
- DOI: http://doi.org/10.13004/kjnt.2014.10.1.1
Abstract
OBJECTIVE
This study describes a method for inducing spinal cord injuries in dogs by using balloon catheters via laminectomy and the subsequent changes in the electrophysiological response.
METHODS
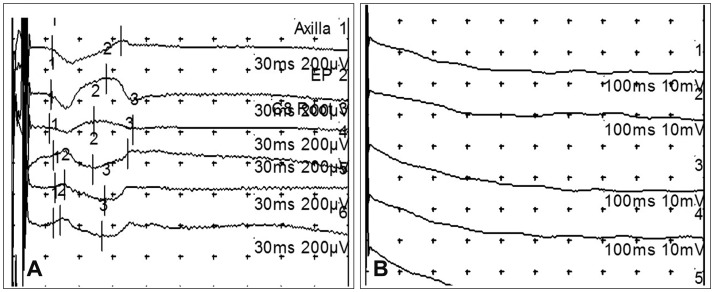
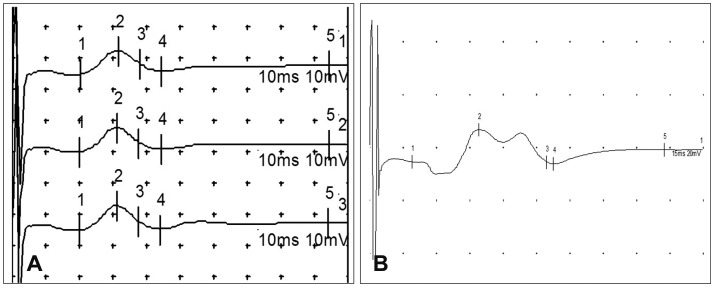
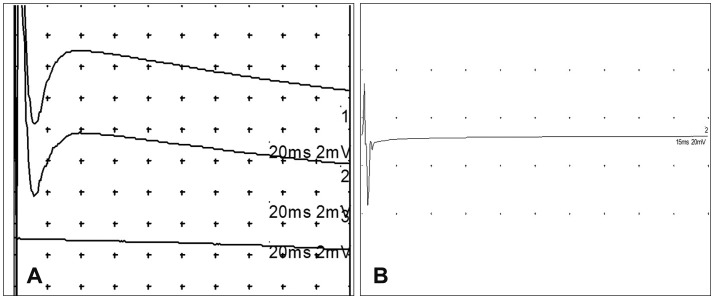
Female Beagle (Orient Bio, Seongnam, Korea) dogs weighing 10 kg at the time of injury were used. Under inhalation anesthesia, a posterior midline approach laminectomy was performed. A silicone balloon catheter (size 6 Fr; Sewoon Medical, Cheonan, Korea) was then inserted into the vertebral canal at the center of T10. The balloon was inflated to the maximum volume for 1, 2, or 3 days. Open field testing was performed for evaluating motor functions of the hindlimbs. Motor evoked potentials (MEPs) induced by electrical and magnetic stimulation were recorded before and after spinal cord injury.
RESULTS
Open field testing yielded locomotor scores of 0 or 1 for dogs subjected to compression for 3 days. These dogs showed no obvious improvement throughout the observation period, and the tonus of their hindlimbs was flaccid. In contrast, motor functions of dogs that had experienced compression for 1 or 2 days were variable, and all dogs showed spastic tonus in their hindlimbs. In dogs subjected to after compression for 3 days, electrically stimulated MEPs for the hindlimbs showed a significant amplitude reduction. Further, hindlimb movements were not evoked by magnetic stimulation of the cervical spine and vertex area.
CONCLUSION
Compression for 3 days with a balloon catheter is a safe, reproducible, and reliable method for evaluating electrophysiological changes in a dog model of complete spinal cord injury.
MeSH Terms
Figure
Reference
-
1. Aguilar RM, Steward O. A bilateral cervical contusion injury model in mice: assessment of gripping strength as a measure of forelimb motor function. Exp Neurol. 2010; 221:38–53. PMID: 19815010.
Article2. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21. PMID: 7783230.
Article3. Chiba A, Oshio K, Inase M. Magnetically evoked EMGs in rats. Neurol Res. 2003; 25:87–91. PMID: 12564132.
Article4. Couto PA, Filipe VM, Magalhães LG, Pereira JE, Costa LM, Melo-Pinto P, et al. A comparison of two-dimensional and three-dimensional techniques for the determination of hindlimb kinematics during treadmill locomotion in rats following spinal cord injury. J Neurosci Methods. 2008; 173:193–200. PMID: 18606186.
Article5. Estenne M, Pinet C, De Troyer A. Abdominal muscle strength in patients with tetraplegia. Am J Respir Crit Care Med. 2000; 161(3 Pt 1):707–712. PMID: 10712311.
Article6. Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, et al. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005; 14:171–180. PMID: 15795171.
Article7. Jeffery ND, Lakatos A, Franklin RJ. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma. 2005; 22:1282–1293. PMID: 16305316.
Article8. Kamida T, Fujiki M, Hori S, Isono M. Conduction pathways of motor evoked potentials following transcranial magnetic stimulation: a rodent study using a "figure-8" coil. Muscle Nerve. 1998; 21:722–731. PMID: 9585325.
Article9. Kang SK, Shin MJ, Jung JS, Kim YG, Kim CH. Autologous adipose tissue-derived stromal cells for treatment of spinal cord injury. Stem Cells Dev. 2006; 15:583–594. PMID: 16978061.
Article10. Khan M, Griebel R, Rozdilsky B, Politis M. Hemorrhagic changes in experimental spinal cord injury models. Can J Neurol Sci. 1985; 12:259–262. PMID: 4052887.
Article11. K L. The Cerebral Cortex of the Rat. Bryan Kolb and Richard C. Tees, Eds. MIT Press, Cambridge, MA, 1990. xii, 645 pp., illus. Paper, $35. A Bradford Book. Science. 1990; 250:1457.12. Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, et al. Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci. 2007; 8:275–282. PMID: 17679775.
Article13. Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, et al. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002; 177:575–580. PMID: 12429203.
Article14. Luft AR, Kaelin-Lang A, Hauser TK, Cohen LG, Thakor NV, Hanley DF. Transcranial magnetic stimulation in the rat. Exp Brain Res. 2001; 140:112–121. PMID: 11500803.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute Spinal Cord Injury after Cervical Nerve Root Block
- The Differentiation of Phase of Spinal Cord Injury Based on the Changes in Gene Expression
- Recent Advances in the Pathophysiology and Treatment of Acute Spinal Cord Injury
- Pre-injury Treatment of Methylprednisolone in Experimental Spinal Cord Injury
- Spinal Cord Injury