J Vet Sci.
2014 Sep;15(3):439-442. 10.4142/jvs.2014.15.3.439.
Quantitative measurement of influenza virus replication using consecutive bronchoalveolar lavage in the lower respiratory tract of a ferret model
- Affiliations
-
- 1Avian Disease Laboratory, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. songcs@konkuk.ac.kr
- 2Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3Department of Veterinary Surgery, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- KMID: 2155629
- DOI: http://doi.org/10.4142/jvs.2014.15.3.439
Abstract
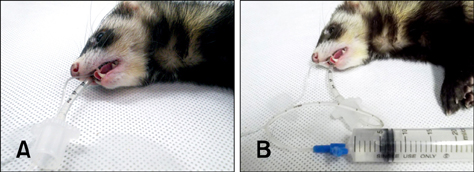
- The ferret is an established animal model of influenza virus infection. Although viral replication in the upper respiratory tract is usually measured with consecutively collected nasal washes, daily evaluation of viral replication in the lung is limited because a large numbers of ferrets need to be sacrificed at consecutive time points. To overcome this limitation, we performed a virus quantification assay using bronchoalveolar lavage (BAL) fluid. This non-invasive BAL technique allows consecutive quantification of virus replication in the lungs of living ferrets. Our method can be used for the longitudinal evaluation of virus tropism in the lower respiratory tract.
Keyword
MeSH Terms
Figure
Reference
-
1. Belser JA, Katz JM, Tumpey TM. The ferret as a model organism to study influenza A virus infection. Dis Model Mech. 2011; 4:575–579.
Article2. Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, Smith J, Vogel FR, Zambon M, Haaheim LR, Wood J. A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respir Viruses. 2009; 3:107–117.
Article3. Ducatez MF, Webb A, Crumpton JC, Webby RJ. Long-term vaccine-induced heterologous protection against H5N1 influenza viruses in the ferret model. Influenza Other Respir Viruses. 2012; 7:506–512.
Article4. Enserink M. Pandemic influenza. Ferrets shed light on new virus's severity and spread. Science. 2009; 325:17.
Article5. Lee YN, Lee DH, Park JK, Yuk SS, Kwon JH, Nahm SS, Lee JB, Park SY, Choi IS, Song CS. Experimental infection and natural contact exposure of ferrets with canine influenza virus (H3N2). J Gen Virol. 2013; 94:293–297.
Article6. Maher JA, DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim (NY). 2004; 33:50–53.
Article7. Maines TR, Jayaraman A, Belser JA, Wadford DA, Pappas C, Zeng H, Gustin KM, Pearce MB, Viswanathan K, Shriver ZH, Raman R, Cox NJ, Sasisekharan R, Katz JM, Tumpey TM. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009; 325:484–487.
Article8. Munster VJ, de Wit E, van den Brand JMA, Herfst S, Schrauwen EJA, Bestebroer TM, van de Vijver D, Boucher CA, Koopmans M, Rimmelzwaan GF, Kuiken T, Osterhaus ADME, Fouchier RAM. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009; 325:481–483.
Article9. Spackman E, Senne DA, Bulaga LL, Myers TJ, Perdue ML, Garber LP, Lohman K, Daum LT, Suarez DL. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003; 47:3 Suppl. 1079–1082.
Article10. Svitek N, von Messling V. Early cytokine mRNA expression profiles predict Morbillivirus disease outcome in ferrets. Virology. 2007; 362:404–410.
Article11. Veldhuis Kroeze EJB, Stittelaar KJ, Teeuwsen VJ, Dijkshoorn ML, van Amerongen G, de Waal L, Kuiken T, Krestin GP, Hinkula J, Osterhaus ADME. Consecutive CT in vivo lung imaging as quantitative parameter of influenza vaccine efficacy in the ferret model. Vaccine. 2012; 30:7391–7394.
Article12. Veldhuis Kroeze EJB, van Amerongen G, Dijkshoorn ML, Simon JH, de Waal L, Hartmann IJC, Krestin GP, Kuiken T, Osterhaus ADME, Stittelaar KJ. Pulmonary pathology of pandemic influenza A/H1N1 virus (2009)-infected ferrets upon longitudinal evaluation by computed tomography. J Gen Virol. 2011; 92:1854–1858.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Novel Influenza A (H1N1) Virus Pneumonia Complicated Pnemomediastinum and Subcutenous Emphysema
- Changes of the cellularities in the bronchoalveolar lavage fluid of the experimental silicosis
- Recovery of respiratory syncytial virus, adenovirus, influenza virus , and parainfluenza virus from nasopharyngeal aspirates from children with acute respiratory tract infections
- Epidemiological Analysis of Viral Respiratory Infections and Comparison of Isolation Rate of Various Clinical Specimens
- A Case of Mycobacterium abscessus Lung Disease in a Patient with H1N1 Influenza Pneumonia