Korean J Radiol.
2015 Aug;16(4):919-928. 10.3348/kjr.2015.16.4.919.
Detection of Myocardial Metabolic Abnormalities by 18F-FDG PET/CT and Corresponding Pathological Changes in Beagles with Local Heart Irradiation
- Affiliations
-
- 1Nursing College of Shanxi Medical University, Taiyuan 030001, China.
- 2Department of Nuclear Medicine, First Hospital of Shanxi Medical University, Taiyuan 030001, China. lisj_nm1@sohu.com
- 3Department of Cardiology, First Hospital of Shanxi Medical University, Taiyuan 030001, China.
- 4Department of Radiological and Environmental Medicine, China Institute for Radiation Protection, Taiyuan 030006, China.
- KMID: 2155568
- DOI: http://doi.org/10.3348/kjr.2015.16.4.919
Abstract
OBJECTIVE
To determine the efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in the detection of radiation-induced myocardial damage in beagles by comparing two pre-scan preparation protocols as well as to determine the correlation between abnormal myocardial FDG uptake and pathological findings.
MATERIALS AND METHODS
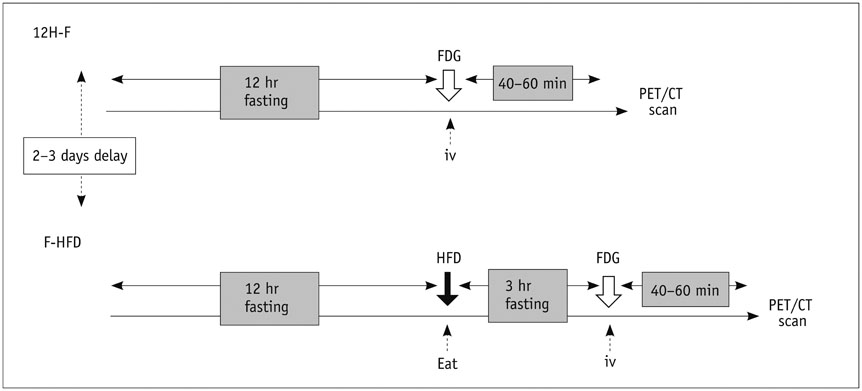
The anterior myocardium of 12 beagles received radiotherapy locally with a single X-ray dose of 20 Gy. 18F-FDG cardiac PET/CT was performed at baseline and 3 months after radiation. Twelve beagles underwent two protocols before PET/CT: 12 hours of fasting (12H-F), 12H-F followed by a high-fat diet (F-HFD). Regions of interest were drawn on the irradiation and the non-irradiation fields to obtain their maximal standardized uptake values (SUVmax). Then the ratio of the SUV of the irradiation to the non-irradiation fields (INR) was computed. Histopathological changes were identified by light and electron microscopy.
RESULTS
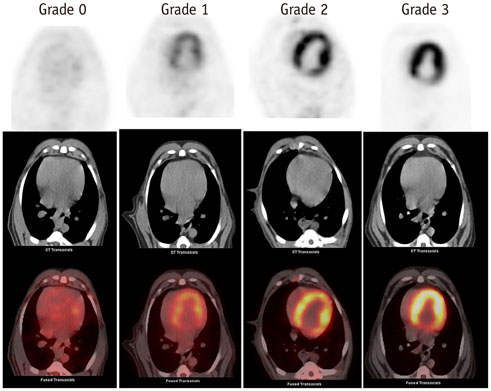
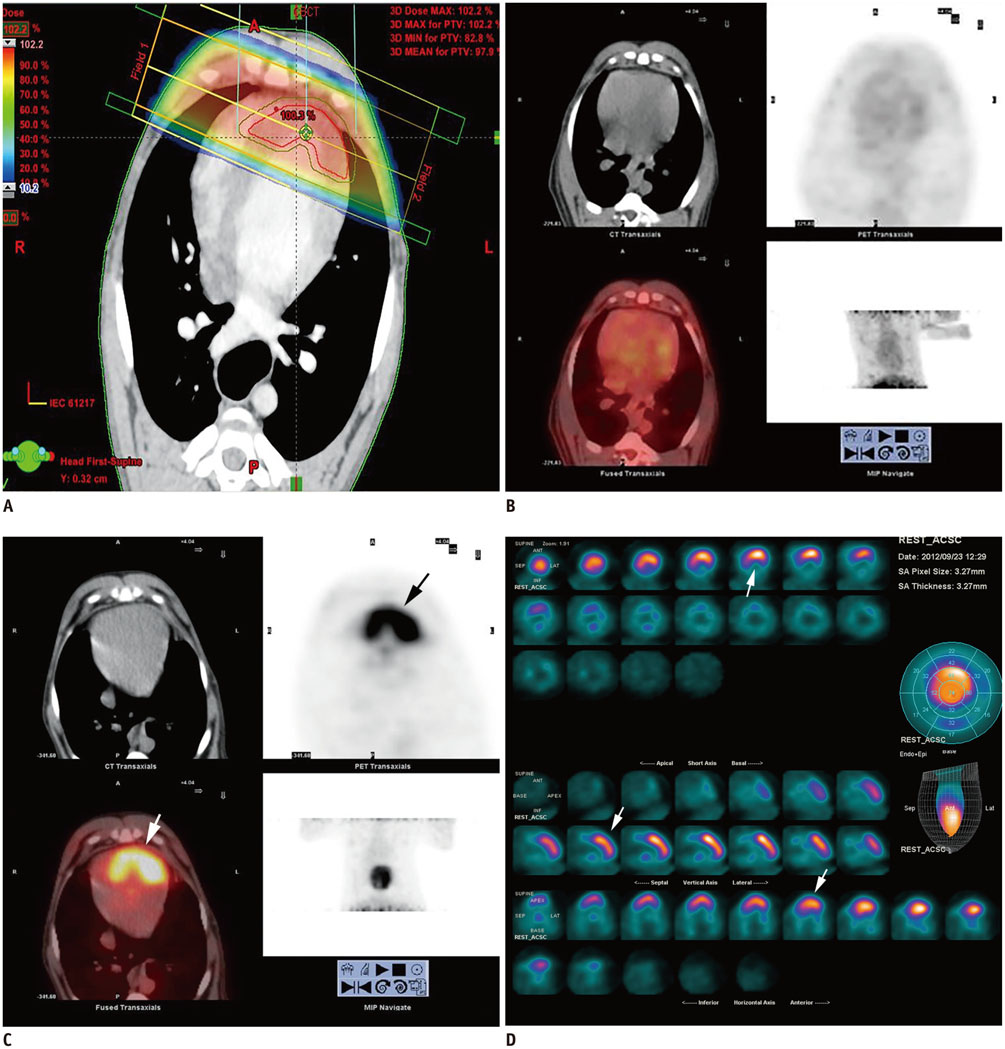
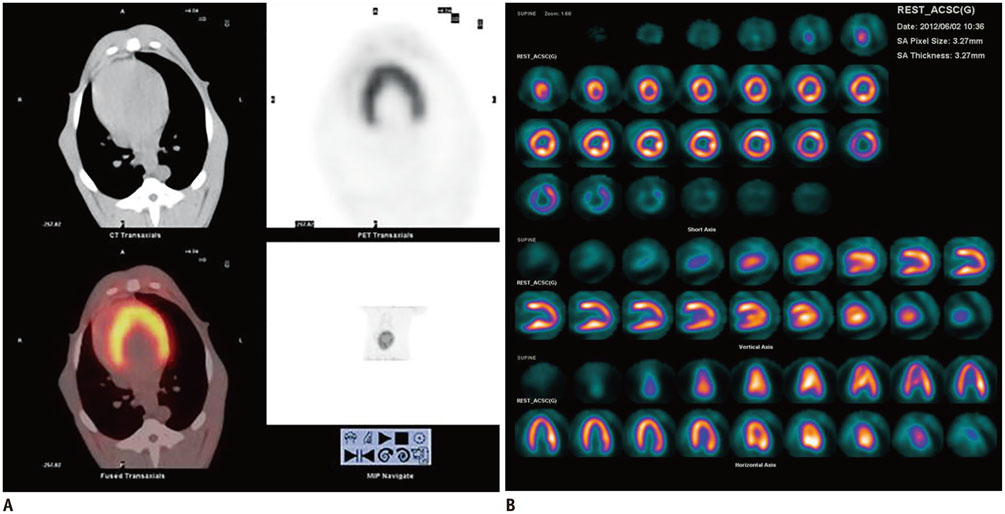
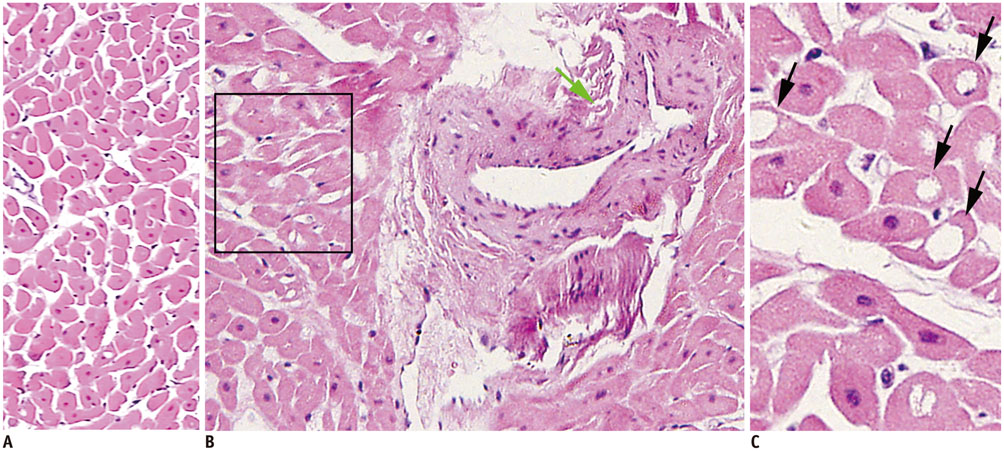
Using the 12H-F protocol, the average INRs were 1.18 +/- 0.10 and 1.41 +/- 0.18 before and after irradiation, respectively (p = 0.021). Using the F-HFD protocol, the average INRs were 0.99 +/- 0.15 and 2.54 +/- 0.43, respectively (p < 0.001). High FDG uptake in irradiation field was detected in 33.3% (4/12) of 12H-F protocol and 83.3% (10/12) of F-HFD protocol in visual analysis, respectively (p = 0.031). The pathology of the irradiated myocardium showed obvious perivascular fibrosis and changes in mitochondrial vacuoles.
CONCLUSION
High FDG uptake in an irradiated field may be related with radiation-induced myocardial damage resulting from microvascular damage and mitochondrial injury. An F-HFD preparation protocol used before obtaining PET/CT can improve the sensitivity of the detection of cardiotoxicity associated with radiotherapy.
MeSH Terms
Figure
Reference
-
1. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 2000; 355:1757–1177.2. Aleman BM, van den Belt-Dusebou AW, De Bruin ML, van't Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007; 109:1878–1886.3. Lally BE, Detterbeck FC, Geiger AM, Thomas CR Jr, Machtay M, Miller AA, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007; 110:911–917.4. Hardy D, Liu CC, Cormier JN, Xia R, Du XL. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol. 2010; 21:1825–1833.5. Mukherjee S, Aston D, Minett M, Brewster AE, Crosby TD. The significance of cardiac doses received during chemoradiation of oesophageal and gastro-oesophageal junctional cancers. Clin Oncol (R Coll Radiol). 2003; 15:115–120.6. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011; 378:1707–1716.7. Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–2106.8. Townsend DW, Carney JP, Yap JT, Hall NC. PET/CT today and tomorrow. J Nucl Med. 2004; 45:4S–14S.9. Jingu K, Kaneta T, Nemoto K, Ichinose A, Oikawa M, Takai Y, et al. The utility of 18F-fluorodeoxyglucose positron emission tomography for early diagnosis of radiation-induced myocardial damage. Int J Radiat Oncol Biol Phys. 2006; 66:845–851.10. Zöphel K, Hölzel C, Dawel M, Hölscher T, Evers C, Kotzerke J. PET/CT demonstrates increased myocardial FDG uptake following irradiation therapy. Eur J Nucl Med Mol Imaging. 2007; 34:1322–1323.11. Unal K, Unlu M, Akdemir O, Akmansu M. 18F-FDG PET/CT findings of radiotherapy-related myocardial changes in patients with thoracic malignancies. Nucl Med Commun. 2013; 34:855–859.12. Inglese E, Leva L, Matheoud R, Sacchetti G, Secco C, Gandolfo P, et al. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J Nucl Med. 2007; 48:1662–1669.13. Fragasso G, Lucignani G, Fazio F. Nonuniformity in myocardial accumulation of fluorine-18-fluorodeoxyglucose in normal fasted humans. J Nucl Med. 1991; 32:1832–1833.14. Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008; 190:W151–W156.15. Fukuchi K, Ohta H, Matsumura K, Ishida Y. Benign variations and incidental abnormalities of myocardial FDG uptake in the fasting state as encountered during routine oncology positron emission tomography studies. Br J Radiol. 2007; 80:3–11.16. Kobayashi Y, Kumita S, Fukushima Y, Ishihara K, Suda M, Sakurai M. Significant suppression of myocardial (18)F-fluorodeoxyglucose uptake using 24-h carbohydrate restriction and a low-carbohydrate, high-fat diet. J Cardiol. 2013; 62:314–319.17. Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011; 18:926–936.18. Frayn KN. The glucose-fatty acid cycle: a physiological perspective. Biochem Soc Trans. 2003; 31(Pt 6):1115–1119.19. Kaneta T, Hakamatsuka T, Takanami K, Yamada T, Takase K, Sato A, et al. Evaluation of the relationship between physiological FDG uptake in the heart and age, blood glucose level, fasting period, and hospitalization. Ann Nucl Med. 2006; 20:203–208.20. de Groot M, Meeuwis AP, Kok PJ, Corstens FH, Oyen WJ. Influence of blood glucose level, age and fasting period on non-pathological FDG uptake in heart and gut. Eur J Nucl Med Mol Imaging. 2005; 32:98–101.21. Krueger EA, Schipper MJ, Koelling T, Marsh RB, Butler JB, Pierce LJ. Cardiac chamber and coronary artery doses associated with postmastectomy radiotherapy techniques to the chest wall and regional nodes. Int J Radiat Oncol Biol Phys. 2004; 60:1195–1203.22. Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007; 67:10–18.23. Lauk S, Kiszel Z, Buschmann J, Trott KR. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985; 11:801–808.24. Boerma M, Hauer-Jensen M. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract. 2010; 2011:DOI: 10.4061/2011/858262.25. Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010; 97:149–161.26. Umezawa R, Takase K, Jingu K, Takanami K, Ota H, Kaneta T, et al. Evaluation of radiation-induced myocardial damage using iodine-123 β-methyl-iodophenyl pentadecanoic acid scintigraphy. J Radiat Res. 2013; 54:880–889.27. Schultz-Hector S, Kallfass E, Sund M. [Radiation sequelae in the large arteries. A review of clinical and experimental data]. Strahlenther Onkol. 1995; 171:427–436.28. Lauk S, Trott KR. Endothelial cell proliferation in the rat heart following local heart irradiation. Int J Radiat Biol. 1990; 57:1017–1030.29. Schultz-Hector S. Experimental studies on the pathogenesis of damage in the heart. Recent Results Cancer Res. 1993; 130:145–156.30. Vos J, Aarnoudse MW, Dijk F, Lamberts HB. On the cellular origin and development of atheromatous plaques. A light and electron microscopic study of combined X-ray and hypercholesterolemia-induced atheromatosis in the carotid artery of the rabbit. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983; 43:1–16.31. Shankar SM, Marina N, Hudson MM, Hodgson DC, Adams MJ, Landier W, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008; 121:e387–e396.32. Fajardo LF, Stewart JR. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol. 1970; 59:299–316.33. Barjaktarovic Z, Schmaltz D, Shyla A, Azimzadeh O, Schulz S, Haagen J, et al. Radiation-induced signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One. 2011; 6:e27811.34. Dogan I, Sezen O, Sonmez B, Zengin AY, Yenilmez E, Yulug E, et al. Myocardial perfusion alterations observed months after radiotherapy are related to the cellular damage. Nuklearmedizin. 2010; 49:209–215.35. Bateman TM. Advantages and disadvantages of PET and SPECT in a busy clinical practice. J Nucl Cardiol. 2012; 19:Suppl 1. S3–S11.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of 18F-FDG PET/CT in Second Primary Cancer
- Supraclavicular Lymph Node Metastasis from Various Malignancies: Assessment with 18F-Fluorodeoxyglucose Positron Emission Tomography/CT, Contrast-Enhanced CT and Ultrasound
- Nodular Fasciitis Mimicking Malignant Tumor on 18F-FDG PET/CT
- The Role of 18F-FDG PET/CT in the Evaluation of Gastric Cancer Recurrence after Curative Gastrectomy
- Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography and Magnetic Resonance Imaging for Adverse Local Tissue Reactions near Metal Implants after Total Hip Arthroplasty: A Preliminary Report