Korean J Radiol.
2015 Aug;16(4):906-913. 10.3348/kjr.2015.16.4.906.
Clinical Implications of Sulcal Enhancement on Postcontrast Fluid Attenuated Inversion Recovery Images in Patients with Acute Stroke Symptoms
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam 463-707, Korea. xmida@hanmail.net
- 2Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, Seoul 130-701, Korea.
- KMID: 2155566
- DOI: http://doi.org/10.3348/kjr.2015.16.4.906
Abstract
OBJECTIVE
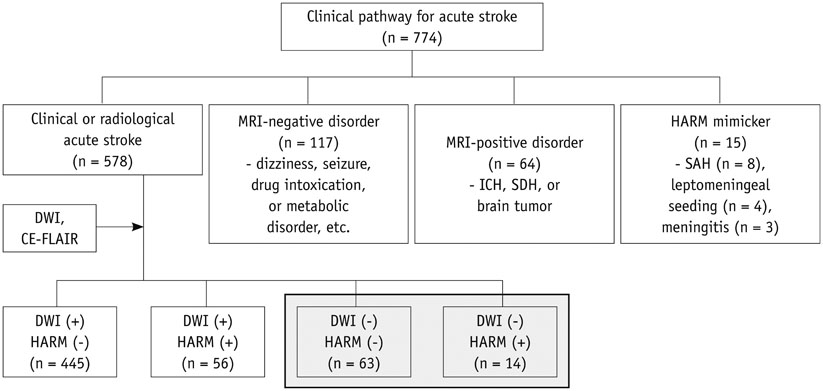
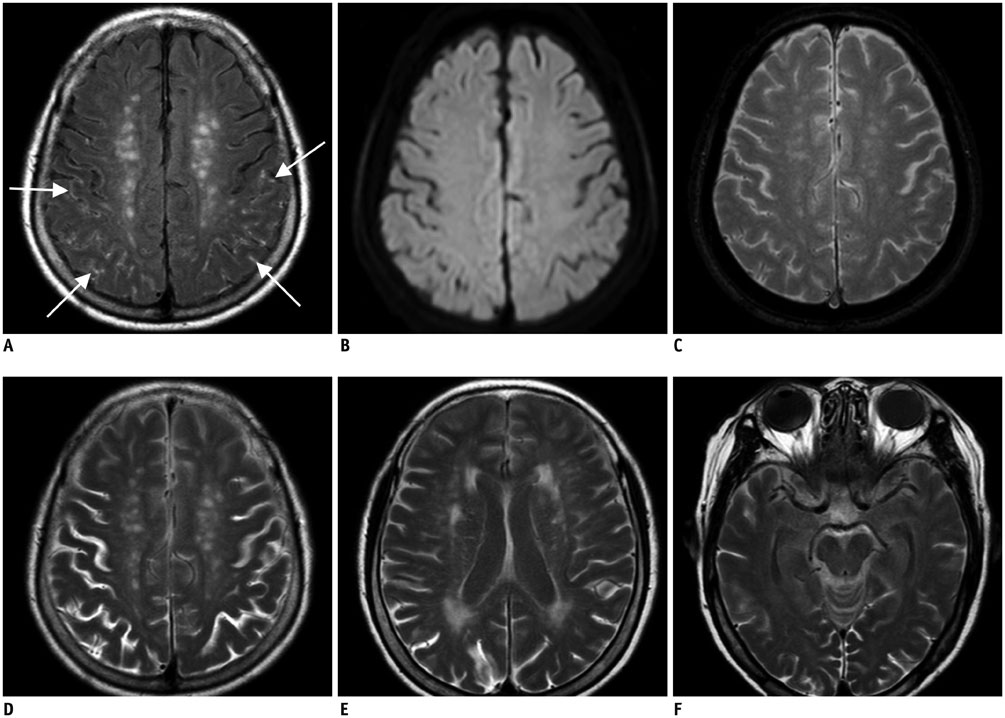
Hyperintense acute reperfusion marker (HARM) without diffusion abnormalities is occasionally found in patients with an acute stroke. This study was to determine the prevalence and clinical implications of HARM without diffusion abnormalities.
MATERIALS AND METHODS
There was a retrospective review of magnetic resonance images 578 patients with acute strokes and identified those who did not have acute infarction lesions, as mapped by diffusion-weighted imaging (DWI). These patients were classified into an imaging-negative stroke and HARM without diffusion abnormalities groups, based on the DWI findings and postcontrast fluid attenuated inversion recovery images. The National Institutes of Health Stroke Scale (NIHSS) scores at admission, 1 day, and 7 days after the event, as well as clinical data and risk factors, were compared between the imaging-negative stroke and HARM without diffusion abnormalities groups.
RESULTS
Seventy-seven acute stroke patients without any DWI abnormalities were found. There were 63 patients with an imaging-negative stroke (accounting for 10.9% of 578) and 13 patients with HARM without diffusion abnormalities (accounting for 2.4% of 578). The NIHSS scores at admission were higher in HARM without diffusion abnormalities group than in the imaging-negative stroke group (median, 4.5 vs. 1.0; p < 0.001), but the scores at 7 days after the event were not significantly different between the two groups (median, 0 vs. 0; p = 1). The patients with HARM without diffusion abnormalities were significantly older, compared with patients with an imaging-negative stroke (mean, 73.1 years vs. 55.9 years; p < 0.001).
CONCLUSION
Patients with HARM without diffusion abnormalities are older and have similarly favorable short-term neurological outcomes, compared with the patients with imaging-negative stroke.
Keyword
MeSH Terms
Figure
Reference
-
1. Köhrmann M, Struffert T, Frenzel T, Schwab S, Doerfler A. The hyperintense acute reperfusion marker on fluid-attenuated inversion recovery magnetic resonance imaging is caused by gadolinium in the cerebrospinal fluid. Stroke. 2012; 43:259–261.2. Bonzano L, Roccatagliata L, Levrero F, Mancardi GL, Sardanelli F. In vitro investigation of poor cerebrospinal fluid suppression on fluid-attenuated inversion recovery images in the presence of a gadolinium-based contrast agent. Magn Reson Med. 2008; 60:220–223.3. Ewing JR, Knight RA, Nagaraja TN, Yee JS, Nagesh V, Whitton PA, et al. Patlak plots of Gd-DTPA MRI data yield blood-brain transfer constants concordant with those of 14C-sucrose in areas of blood-brain opening. Magn Reson Med. 2003; 50:283–292.4. Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004; 35:11 Suppl 1. 2659–2661.5. Dechambre SD, Duprez T, Grandin CB, Lecouvet FE, Peeters A, Cosnard G. High signal in cerebrospinal fluid mimicking subarachnoid haemorrhage on FLAIR following acute stroke and intravenous contrast medium. Neuroradiology. 2000; 42:608–611.6. Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004; 56:468–477.7. Henning EC, Latour LL, Warach S. Verification of enhancement of the CSF space, not parenchyma, in acute stroke patients with early blood-brain barrier disruption. J Cereb Blood Flow Metab. 2008; 28:882–886.8. Rozanski M, Ebinger M, Schmidt WU, Hotter B, Pittl S, Heuschmann PU, et al. Hyperintense acute reperfusion marker on FLAIR is not associated with early haemorrhagic transformation in the elderly. Eur Radiol. 2010; 20:2990–2996.9. Ogami R, Nakahara T, Hamasaki O, Araki H, Kurisu K. Cerebrospinal fluid enhancement on fluid attenuated inversion recovery images after carotid artery stenting with neuroprotective balloon occlusions: hemodynamic instability and blood-brain barrier disruption. Cardiovasc Intervent Radiol. 2011; 34:936–941.10. Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010; 41:e123–e128.11. Merino JG, Latour LL, Tso A, Lee KY, Kang DW, Davis LA, et al. Blood-brain barrier disruption after cardiac surgery. AJNR Am J Neuroradiol. 2013; 34:518–523.12. Kim DW, Moon Y, Gee Noh H, Choi JW, Oh J. Blood-brain barrier disruption is involved in seizure and hemianopsia in nonketotic hyperglycemia. Neurologist. 2011; 17:164–166.13. Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008; 25:338–343.14. Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging. 2009; 30:337–352.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversible Cerebral Vasoconstriction Syndrome Presenting as Transient Vessel Wall Enhancement on Contrast-Enhanced Fluid-Attenuated Inversion Recovery Images: A Case Report and Literature Review
- Hyperintense Vessels on FLAIR MRI in Patients With Acute Middle Cerebral Artery Infarction Revealed Pial Collateral on Cerebral Angiography
- Importance of Contrast-Enhanced Fluid-Attenuated Inversion Recovery Magnetic Resonance Imaging in Various Intracranial Pathologic Conditions
- Isolated Leptomeningeal Enhancement in Anti-N-Methyl D-Aspartate Receptor Encephalitis: The Diagnostic Value of Contrast-Enhanced Fluid-Attenuated Inversion Recovery Imaging
- Transient Sulcal Hyperintensity on Fluid-Attenuated Inversion Recovery MRI in Postpartum Cerebral Angiopathy Induced by Bromocriptine