Electrolyte Blood Press.
2015 Dec;13(2):52-57. 10.5049/EBP.2015.13.2.52.
A Case Report of Familial Renal Hypouricemia Confirmed by Genotyping of SLC22A12, and a Literature Review
- Affiliations
-
- 1Department of Internal Medicine, Kyung Hee University College of Medicine, Seoul, Korea. kidwon@khmc.or.kr
- KMID: 2152087
- DOI: http://doi.org/10.5049/EBP.2015.13.2.52
Abstract
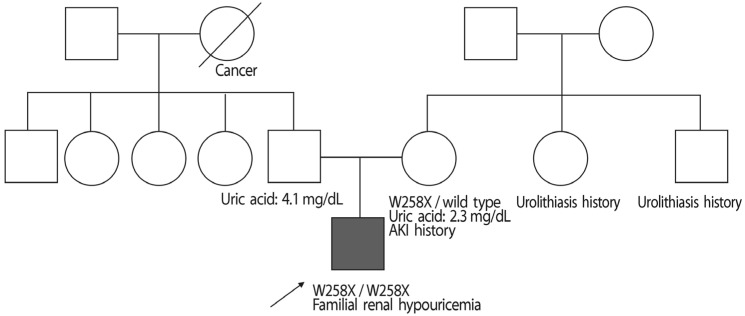
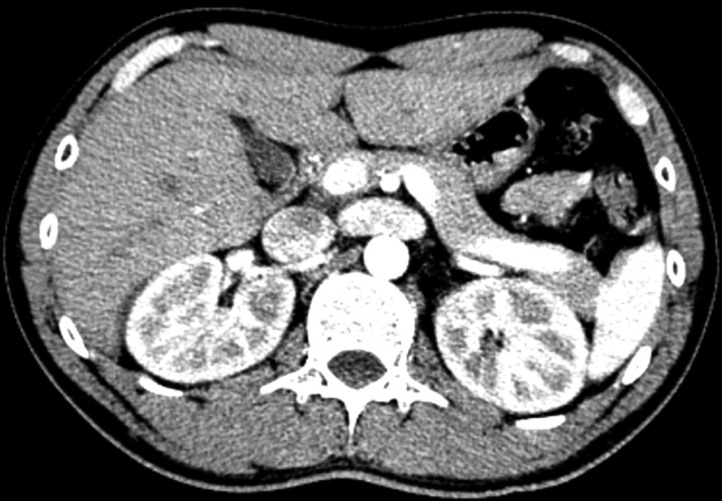
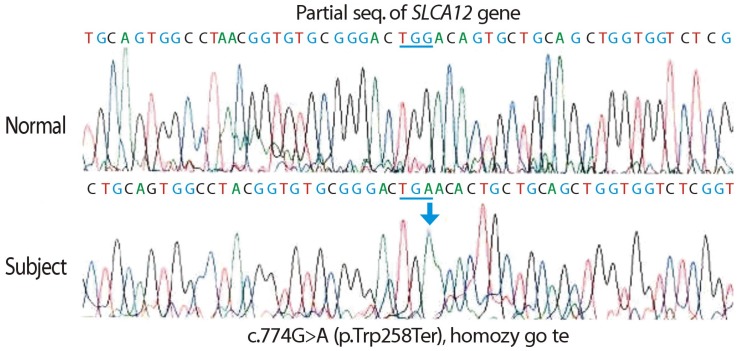
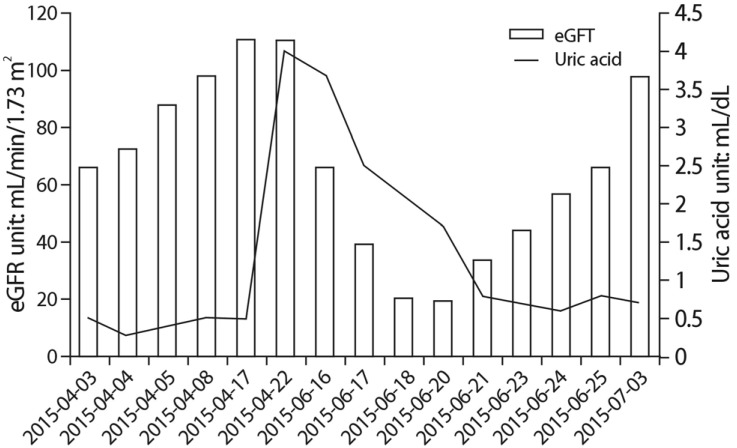
- A 24-year-old male visited our hospital because of pain in both flanks. His biochemistry profile showed an elevated serum creatinine level and low serum uric acid level. History taking revealed that he had undertaken exercise prior to the acute kidney injury (AKI) event, and he stated that family members had a history of urolithiasis. His renal profile improved after hydration and supportive care during hospitalization. Although the patient was subsequently admitted again due to AKI, his status recovered with similar treatment. Since the diagnosis of the patient was familial renal hypouricemia with exercise-induced AKI, we performed genotyping of SLC22A12, which encodes human urate transporter 1. The diagnosis was confirmed by the detection of a homozygous mutation of W258X. We herein, report a case of familial renal hypouricemia confirmed by genotyping of SLC22A12, and review the relevant literature.
MeSH Terms
Figure
Reference
-
1. Sperling O. Hereditary renal hypouricemia. Mol Genet Metab. 2006; 89(1-2):14–18. PMID: 16678460.
Article2. Shen H, Feng C, Jin X, et al. Recurrent exercise-induced acute kidney injury by idiopathic renal hypouricemia with a novel mutation in the SLC2A9 gene and literature review. BMC Pediatr. 2014; 14:73. PMID: 24628802.
Article3. Ishikawa I, Sakurai Y, Masuzaki S, Sugishita N, Shinoda A, Shikura N. Exercise-induced acute renal failure in 3 patients with renal hypouricemia. Nihon Jinzo Gakkai Shi. 1990; 32(8):923–992. PMID: 2250410.4. Ichida K. Clinical and Molecular Analysis of Patients with Renal Hypouricemia in Japan-Influence of URAT1 Gene on Urinary Urate Excretion. J Am Soc Nephrol. 2004; 15(1):164–173. PMID: 14694169.
Article5. Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002; 417(6887):447–452. PMID: 12024214.6. Dinour D, Gray NK, Campbell S, et al. Homozygous SLC2A9 mutations cause severe renal hypouricemia. J Am Soc Nephrol. 2010; 21(1):64–72. PMID: 19926891.
Article7. Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008; 40(4):437–442. PMID: 18327257.
Article8. Kim YH, Cho JT. A case of exercise-induced acute renal failure with G774A mutation in SCL22A12 causing renal hypouricemia. J Korean Med Sci. 2011; 26(9):1238–1240. PMID: 21935282.
Article9. Kim AJ, Park SY, Jung JY, Chang JH, Lee HH, Chung WY, Ro H. A Case of Recurrent Exercise-Induced Acute Renal Failure and Renal Hypouricemia with R90H Mutation in a SCL22A12 Gene. Yeungnam Univ J Med. 2012; 29(2):150–152.
Article10. Cheong HI, Kang JH, Lee JH, et al. Mutational analysis of idiopathic renal hypouricemia in Korea. Pediatr Nephrol. 2005; 20(7):886–890. PMID: 15912381.
Article11. Lee JH, Choi JH, Park YS, Yoo HW, Jeong JY. A Case of Idiopathic Renal Hypouricemia with URAT1 Gene Mutation who Showed Persistent Orange-colored Urine. J Korean Soc Pediatr Nephrol. 2006; 10(1):65–71.12. Hagos Y, Stein D, Ugele B, Burckhardt G, Bahn A. Human renal organic anion transporter 4 operates as an asymmetric urate transporter. J Am Soc Nephrol. 2007; 18(2):430–439. PMID: 17229912.
Article13. Bahn A, Hagos Y, Reuter S, et al. Identification of a new urate and high affinity nicotinate transporter, hOAT10 (SLC22A13). J Biol Chem. 2008; 283(24):16332–16341. PMID: 18411268.
Article14. Eraly SA, Vallon V, Rieg T, et al. Multiple organic anion transporters contribute to net renal excretion of uric acid. Physiol Genomics. 2008; 33(2):180–192. PMID: 18270321.
Article15. Murakami T, Kawakami H, Fukuda M, Furukawa S. Patients with renal hypouricemia are prone to develop acute renal failure-why. Clin Nephrol. 1995; 43(3):207–208. PMID: 7774083.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Severe Acute Kidney Injury with Familial Renal Hypouricemia Confirmed by Genotyping of SLC22A12
- A Case of Idiopathic Renal Hypouricemia with SLC22A12 Gene Mutation Showing General Weakness and Incidental Renal Stone
- A case of idiopathic renal hypouricemia
- A Case of Idiopathic Renal Hypouricemia with URAT1 Gene Mutation who Showed Persistent Orange-colored Urine
- A Case of Exercise-induced Acute Renal Failure with G774A Mutation in SCL22A12 Causing Renal Hypouricemia