Investig Magn Reson Imaging.
2015 Dec;19(4):205-211. 10.13104/imri.2015.19.4.205.
Congenital Heart Disease: a Pictorial Illustration of Putting Segmental Approach into Practice
- Affiliations
-
- 1Department of Radiology, Princess Margaret Hospital, Hong Kong. lyc713@ha.org.hk
- 2Department of Radiology, Seoul National University Hospital, Seoul, Korea.
- KMID: 2151766
- DOI: http://doi.org/10.13104/imri.2015.19.4.205
Abstract
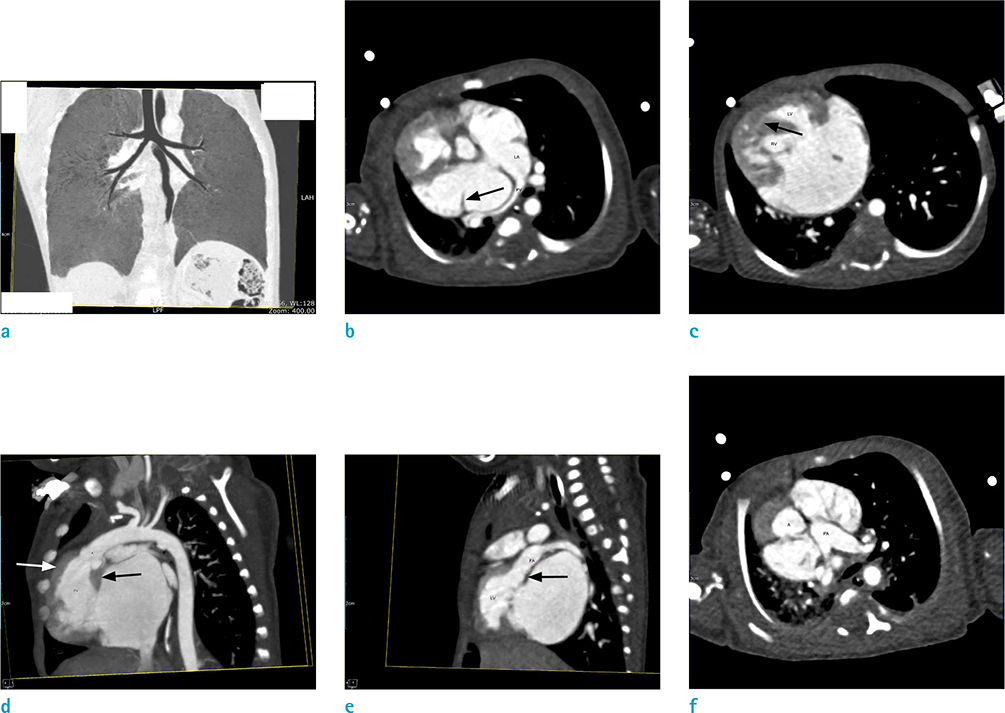
- The human heart is a complex organ in which many complicated congenital defects may happen and some of them require surgical intervention. Due to the vast complexity of varied anatomical presentations, establishing an accurate and consistent nomenclature system is utmost important to facilitate effective communication among pediatric cardiologists, cardiothoracic surgeons and radiologists. The Van Praagh segmental approach to the complex congenital heart disease (CHD) was developed in the 1960s and has been used widely as the language for describing complex anatomy of CHD over the decades. It utilizes a systematic and sequential method to describe the cardiac segments and connections which in turn allows accurate, comprehensive and unambiguous description of CHD. It can also be applied to multiple imaging modalities such as echocardiogram, cardiac CT and MRI. The Van Praagh notation demonstrates a group of three letters, with each letter representative for a key embryologic region of cardiac anatomy: the atria, ventricles and great vessels. By using a 3-steps approach, we can evaluate complex CHD precisely and have no difficulties in communicating with other medial colleague. This pictorial essay revisits the logical steps of segmental approach, followed by a pictorial illustration of its application.
Keyword
MeSH Terms
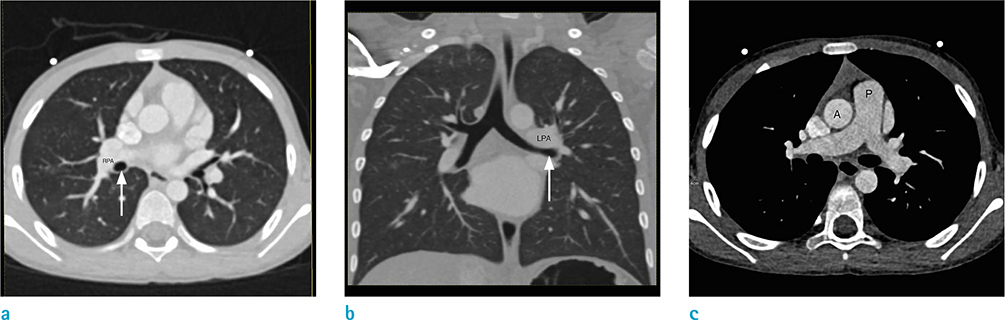
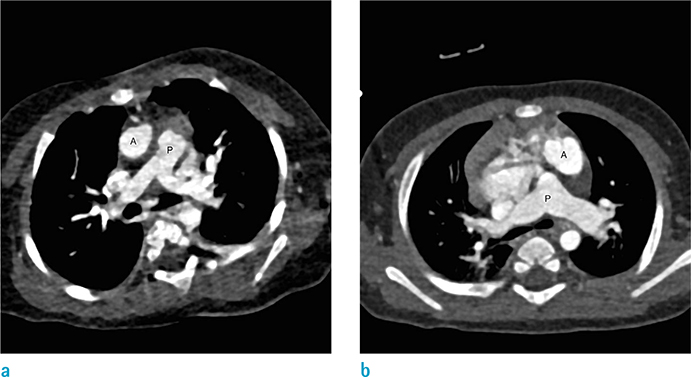
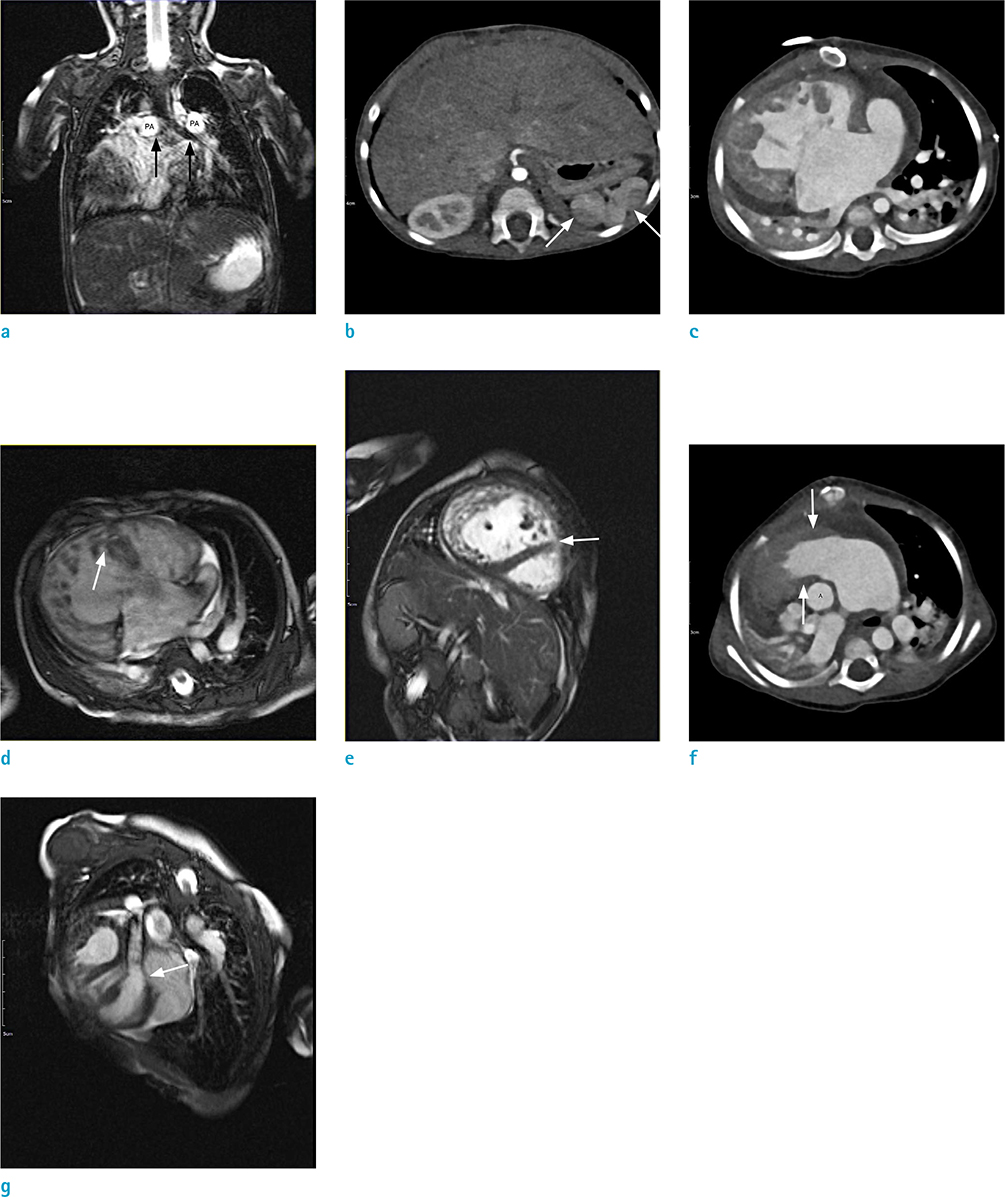
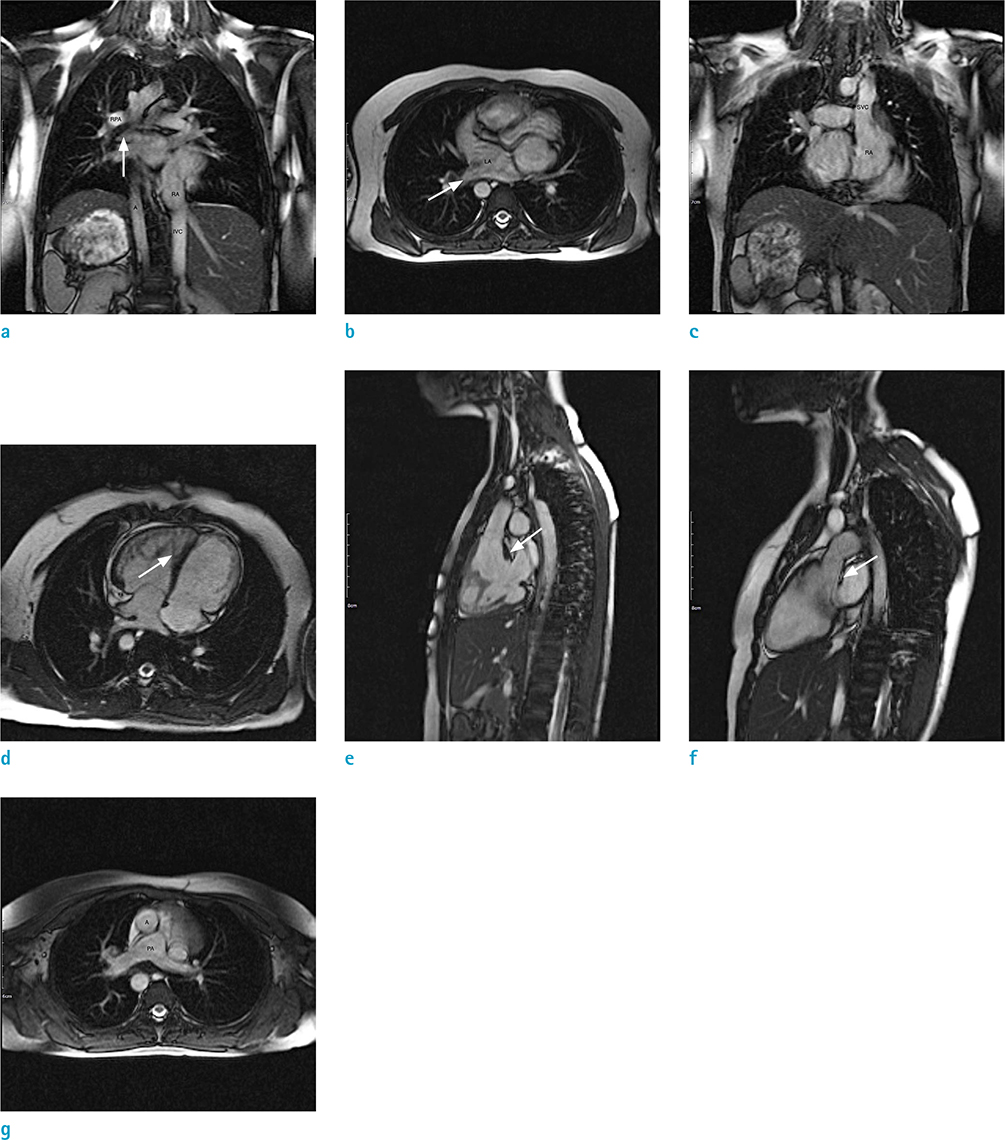
Figure
Reference
-
1. Brandt PW, Calder AL. Cardiac connections: the segmental approach to radiologic diagnosis in congenital heart disease. Curr Probl Diagn Radiol. 1977; 7:1–35.2. Schallert EK, Danton GH, Kardon R, Young DA. Describing congenital heart disease by using three-part segmental notation. Radiographics. 2013; 33:E33–E46.3. Lapierre C, Dery J, Guerin R, Viremouneix L, Dubois J, Garel L. Segmental approach to imaging of congenital heart disease. Radiographics. 2010; 30:397–411.4. Van Praagh R. The segmental approach clarified. Cardiovasc Intervent Radiol. 1984; 7:320–325.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CT-Based Essential Cardiac Anatomy for Radiology Residents to Understand Congenital Heart Disease
- Congenital Anomalies of the Coronary Sinus: A Pictorial Essay
- Echocardiographic Pitfalls for General Physicians in Understanding Adult Congenital Heart Disease
- Effects of Distance and Accuracy on Visual Search in Golf Putting
- Production of Educational Surgical Illustration of Laparoscopic Partial Nephrectomy