Immune Netw.
2011 Dec;11(6):348-357. 10.4110/in.2011.11.6.348.
NDRG2 Promotes GATA-1 Expression through Regulation of the JAK2/STAT Pathway in PMA-stimulated U937 Cells
- Affiliations
-
- 1Department of Biological Science and the Research Center for Women's Disease, Sookmyung Women's University, Seoul 140-742, Korea. jslim@sookmyung.ac.kr
- KMID: 2150719
- DOI: http://doi.org/10.4110/in.2011.11.6.348
Abstract
- BACKGROUND
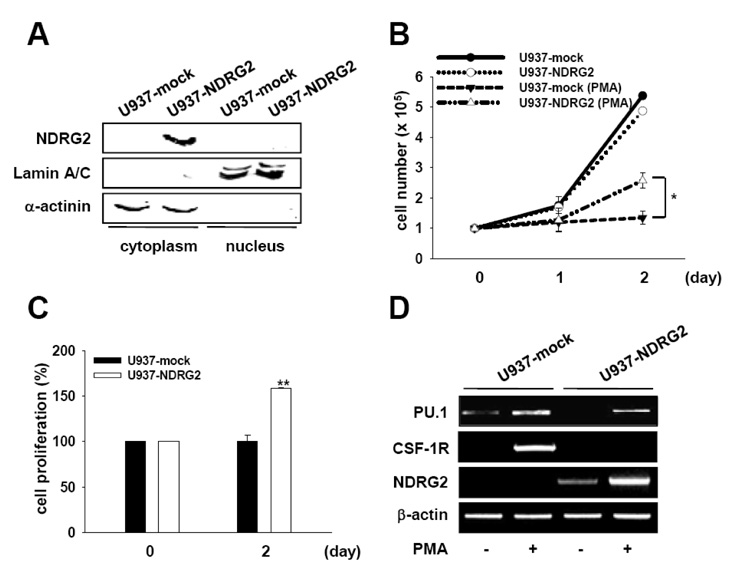
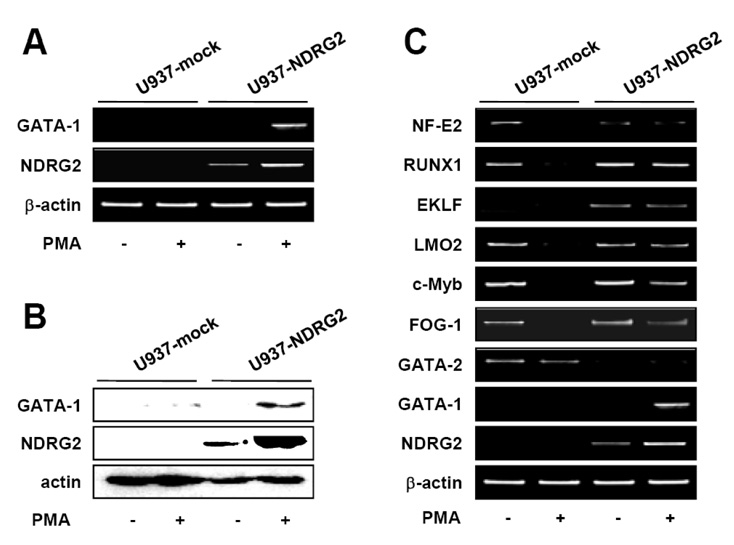
N-myc downstream-regulated gene 2 (NDRG2), a member of a newly described family of differentiation-related genes, has been characterized as a regulator of dendritic cells. However, the role of NDRG2 on the expression and activation of transcription factors in blood cells remains poorly understood. In this study, we investigated the effects of NDRG2 overexpression on GATA-1 expression in PMA-stimulated U937 cells.
METHODS
We generated NDRG2-overexpressing U937 cell line (U937-NDRG2) and treated the cells with PMA to investigate the role of NDRG2 on GATA-1 expression.
RESULTS
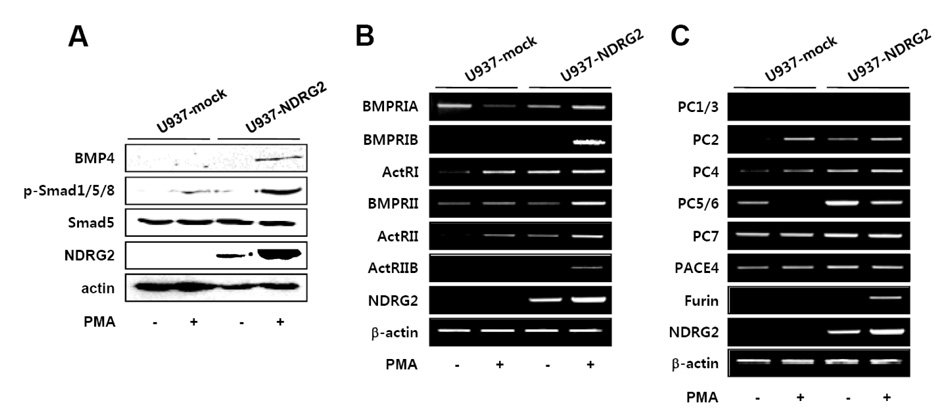
NDRG2 overexpression in U937 cells significantly induced GATA-1 expression in response to PMA stimulation. Interestingly, JAK2/STAT and BMP-4/Smad pathways associated with the induction of GATA-1 were activated in PMA-stimulated U937-NDRG2 cells. We found that the inhibition of JAK2 activation, but not of BMP-4/Smad signaling, can elicit a decrease of PMA-induced GATA-1 expression in U937-NDRG2 cells.
CONCLUSION
The results reveal that NDRG2 promotes the expression of GATA-1 through activation of the JAK2/STAT pathway, but not through the regulation of the BMP-4/Smad pathway in U937 cells. Our findings further suggest that NDRG2 may play a role as a regulator of erythrocyte and megakaryocyte differentiation during hematopoiesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Cantor AB, Orkin SH. Hematopoietic development: a balancing act. Curr Opin Genet Dev. 2001. 11:513–519.
Article2. Arinobu Y, Mizuno S, Chong Y, Shigematsu H, Iino T, Iwasaki H, Graf T, Mayfield R, Chan S, Kastner P, Akashi K. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007. 1:416–427.
Article3. Nerlov C, Graf T. PU.1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev. 1998. 12:2403–2412.
Article4. Ma AC, Fan A, Ward AC, Liongue C, Lewis RS, Cheng SH, Chan PK, Yip SF, Liang R, Leung AY. A novel zebrafish jak2a(V581F) model shared features of human JAK2(V617F) polycythemia vera. Exp Hematol. 2009. 37:1379–1386.
Article5. Ma AC, Ward AC, Liang R, Leung AY. The role of jak2a in zebrafish hematopoiesis. Blood. 2007. 110:1824–1830.
Article6. He D, Chen T, Yang M, Zhu X, Wang C, Cao X, Cai Z. Small Rab GTPase Rab7b promotes megakaryocytic differentiation by enhancing IL-6 production and STAT3-GATA-1 association. J Mol Med (Berl). 2011. 89:137–150.
Article7. Adelman CA, Chattopadhyay S, Bieker JJ. The BMP/BMPR/Smad pathway directs expression of the erythroid-specific EKLF and GATA1 transcription factors during embryoid body differentiation in serum-free media. Development. 2002. 129:539–549.
Article8. Lohmann F, Bieker JJ. Activation of Eklf expression during hematopoiesis by Gata2 and Smad5 prior to erythroid commitment. Development. 2008. 135:2071–2082.
Article9. Anderson GJ, Darshan D. Small-molecule dissection of BMP signaling. Nat Chem Biol. 2008. 4:15–16.
Article10. Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC. Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One. 2008. 3:e2904.
Article11. Ding YL, Xu CW, Wang ZD, Zhan YQ, Li W, Xu WX, Yu M, Ge CH, Li CY, Yang XM. Over-expression of EDAG in the myeloid cell line 32D: induction of GATA-1 expression and erythroid/megakaryocytic phenotype. J Cell Biochem. 2010. 110:866–874.
Article12. de Thonel A, Vandekerckhove J, Lanneau D, Selvakumar S, Courtois G, Hazoume A, Brunet M, Maurel S, Hammann A, Ribeil JA, Zermati Y, Gabet AS, Boyes J, Solary E, Hermine O, Garrido C. HSP27 controls GATA-1 protein level during erythroid cell differentiation. Blood. 2010. 116:85–96.
Article13. Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruïne AP, Baldwin HS, van Engeland M. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010. 24:4153–4166.
Article14. Choi SC, Kim KD, Kim JT, Kim JW, Yoon DY, Choe YK, Chang YS, Paik SG, Lim JS. Expression and regulation of NDRG2 (N-myc downstream regulated gene 2) during the differentiation of dendritic cells. FEBS Lett. 2003. 553:413–418.
Article15. Choi SC, Kim KD, Kim JT, Kim JW, Lee HG, Kim JM, Jang YS, Yoon DY, Kim KI, Yang Y, Cho DH, Lim JS. Expression of human NDRG2 by myeloid dendritic cells inhibits down-regulation of activated leukocyte cell adhesion molecule (ALCAM) and contributes to maintenance of T cell stimulatory activity. J Leukoc Biol. 2008. 83:89–98.
Article16. Deng Y, Yao L, Chau L, Ng SS, Peng Y, Liu X, Au WS, Wang J, Li F, Ji S, Han H, Nie X, Li Q, Kung HF, Leung SY, Lin MC. N-Myc downstream-regulated gene 2 (NDRG2) inhibits glioblastoma cell proliferation. Int J Cancer. 2003. 106:342–347.
Article17. Shon SK, Kim A, Kim JY, Kim KI, Yang Y, Lim JS. Bone morphogenetic protein-4 induced by NDRG2 expression inhibits MMP-9 activity in breast cancer cells. Biochem Biophys Res Commun. 2009. 385:198–203.
Article18. Mitchelmore C, Büchmann-Møller S, Rask L, West MJ, Troncoso JC, Jensen NA. NDRG2: a novel Alzheimer's disease associated protein. Neurobiol Dis. 2004. 16:48–58.
Article19. Takahashi K, Yamada M, Ohata H, Honda K, Yamada M. Ndrg2 promotes neurite outgrowth of NGF-differentiated PC12 cells. Neurosci Lett. 2005. 388:157–162.
Article20. Choi SC, Kim KD, Kim JT, Oh SS, Yoon SY, Song EY, Lee HG, Choe YK, Choi I, Lim JS, Kim JW. NDRG2 is one of novel intrinsic factors for regulation of IL-10 production in human myeloid cell. Biochem Biophys Res Commun. 2010. 396:684–690.
Article21. Lee EB, Kim A, Kang K, Kim H, Lim JS. NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production. Immune Netw. 2010. 10:219–229.
Article22. Carey JO, Posekany KJ, de Vente JE, Pettit GR, Ways DK. Phorbol ester-stimulated phosphorylation of PU.1: association with leukemic cell growth inhibition. Blood. 1996. 87:4316. 4324.
Article23. Goldfarb AN. Megakaryocytic programming by a transcriptional regulatory loop: a circle connecting RUNX1, GATA-1, and P-TEFb. J Cell Biochem. 2009. 107:377–382.
Article24. Wickrema A, Crispino JD. Erythroid and megakaryocytic transformation. Oncogene. 2007. 26:6803–6815.
Article25. Harrison CA, Wiater E, Gray PC, Greenwald J, Choe S, Vale W. Modulation of activin and BMP signaling. Mol Cell Endocrinol. 2004. 225:19–24.
Article26. Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997. 390:465–471.
Article27. Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005. 21:659–693.28. Seidah NG. What lies ahead for the proprotein convertases? Ann N Y Acad Sci. 2011. 1220:149–161.
Article29. Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002. 3:753–766.
Article30. Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. 2009. 284:27157–27166.
Article31. Cui Y, Jean F, Thomas G, Christian JL. BMP-4 is proteolytically activated by furin and/or PC6 during vertebrate embryonic development. EMBO J. 1998. 17:4735–4743.
Article32. Friend C, Scher W, Holland JG, Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971. 68:378–382.
Article33. Rylski M, Welch JJ, Chen YY, Letting DL, Diehl JA, Chodosh LA, Blobel GA, Weiss MJ. GATA-1-mediated proliferation arrest during erythroid maturation. Mol Cell Biol. 2003. 23:5031–5042.
Article34. Bresnick EH, Lee HY, Fujiwara T, Johnson KD, Keles S. GATA switches as developmental drivers. J Biol Chem. 2010. 285:31087–31093.
Article35. Fan C, Ouyang P, Timur AA, He P, You SA, Hu Y, Ke T, Driscoll DJ, Chen Q, Wang QK. Novel roles of GATA1 in regulation of angiogenic factor AGGF1 and endothelial cell function. J Biol Chem. 2009. 284:23331–23343.
Article36. Park Y, Shon SK, Kim A, Kim KI, Yang Y, Cho DH, Lee MS, Lim JS. SOCS1 induced by NDRG2 expression negatively regulates STAT3 activation in breast cancer cells. Biochem Biophys Res Commun. 2007. 363:361–367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NDRG2-mediated Modulation of SOCS3 and STAT3 Activity Inhibits IL-10 Production
- Peroxisome Proliferator-activated Receptor-gamma Inhibits the Activation of STAT3 in Cerulein-stimulated Pancreatic Acinar Cells
- Galangin Regulates Mucin 5AC Gene Expression via the Nuclear Factor-κB Inhibitor α/Nuclear Factor-κB p65 Pathway in Human Airway Epithelial Cells
- Pyronaridine Inhibited MUC5AC Mucin Gene Expression by Regulation of Nuclear Factor Kappa B Signaling Pathway in Human Pulmonary Mucoepidermoid Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells