Cancer Res Treat.
2015 Jul;47(3):343-361. 10.4143/crt.2014.308.
Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hoylim@skku.edu
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Oncology, National Taiwan University Hospital, Taipei, Taiwan.
- 4Department of Internal Medicine, Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan.
- 5National Institute of Cancer Research, National Health Research Institutes, Tainan, Taiwan.
- 6Eli Lilly Interamerica Inc., Buenos Aires, Argentina.
- 7Eli Lilly Korea Ltd., Seoul, Korea.
- KMID: 2148483
- DOI: http://doi.org/10.4143/crt.2014.308
Abstract
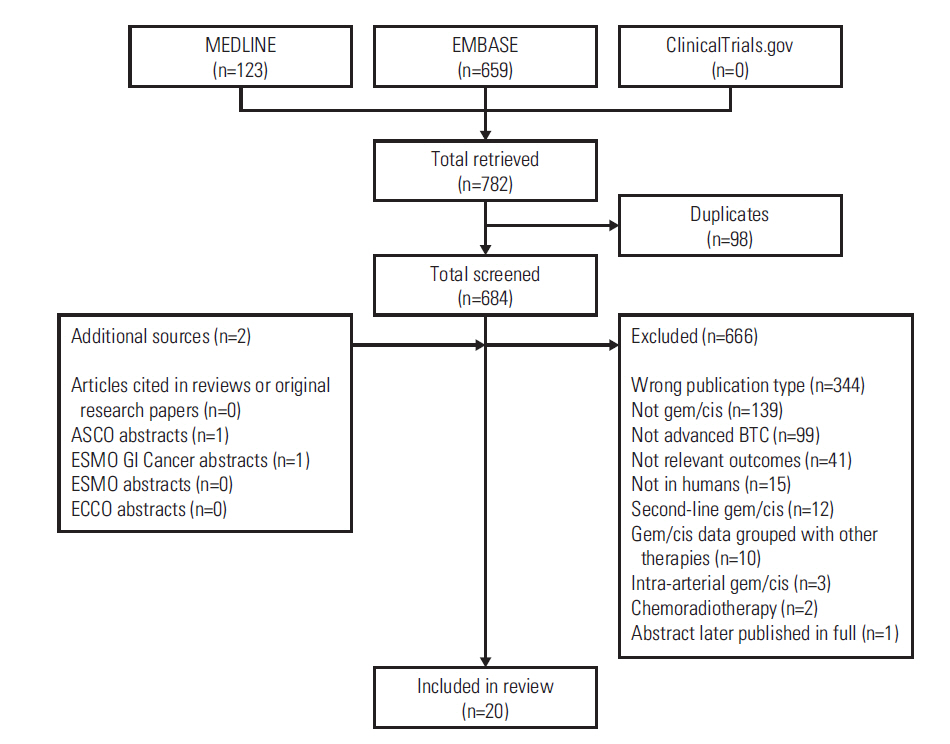
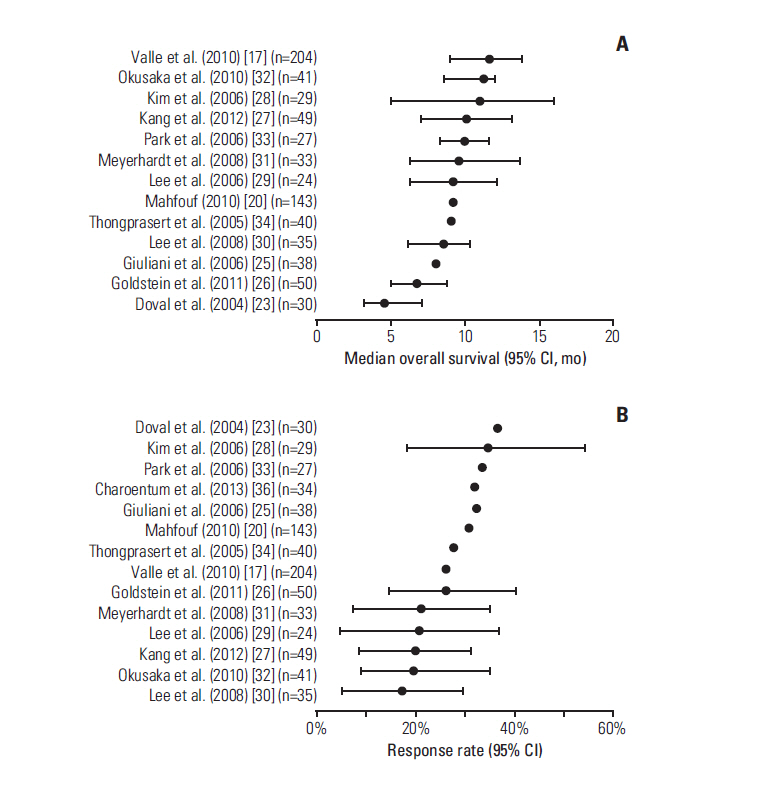
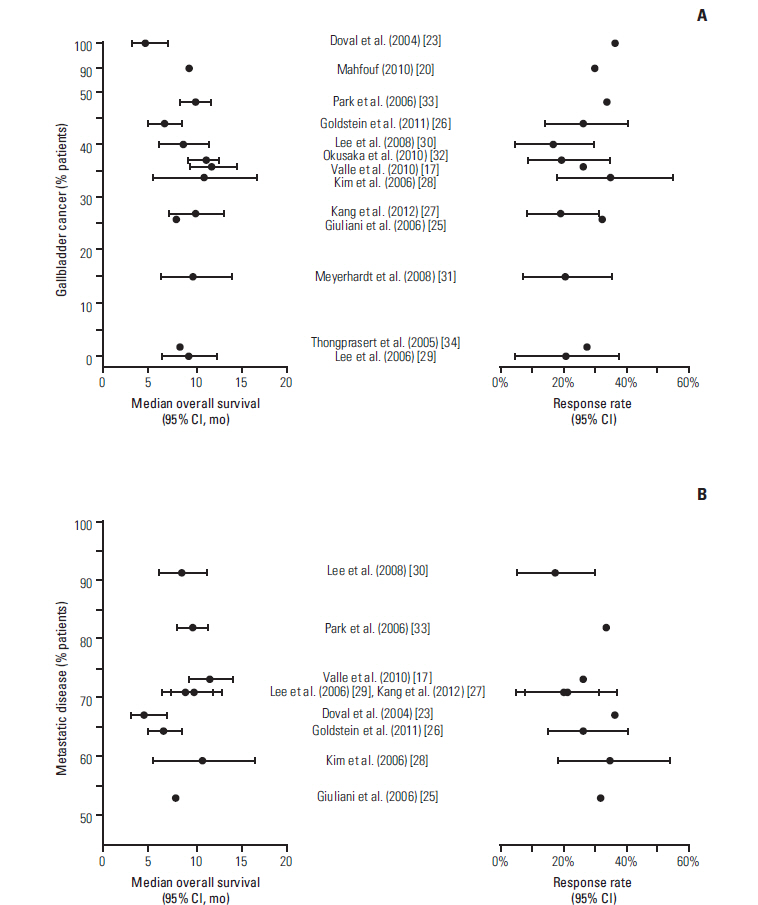
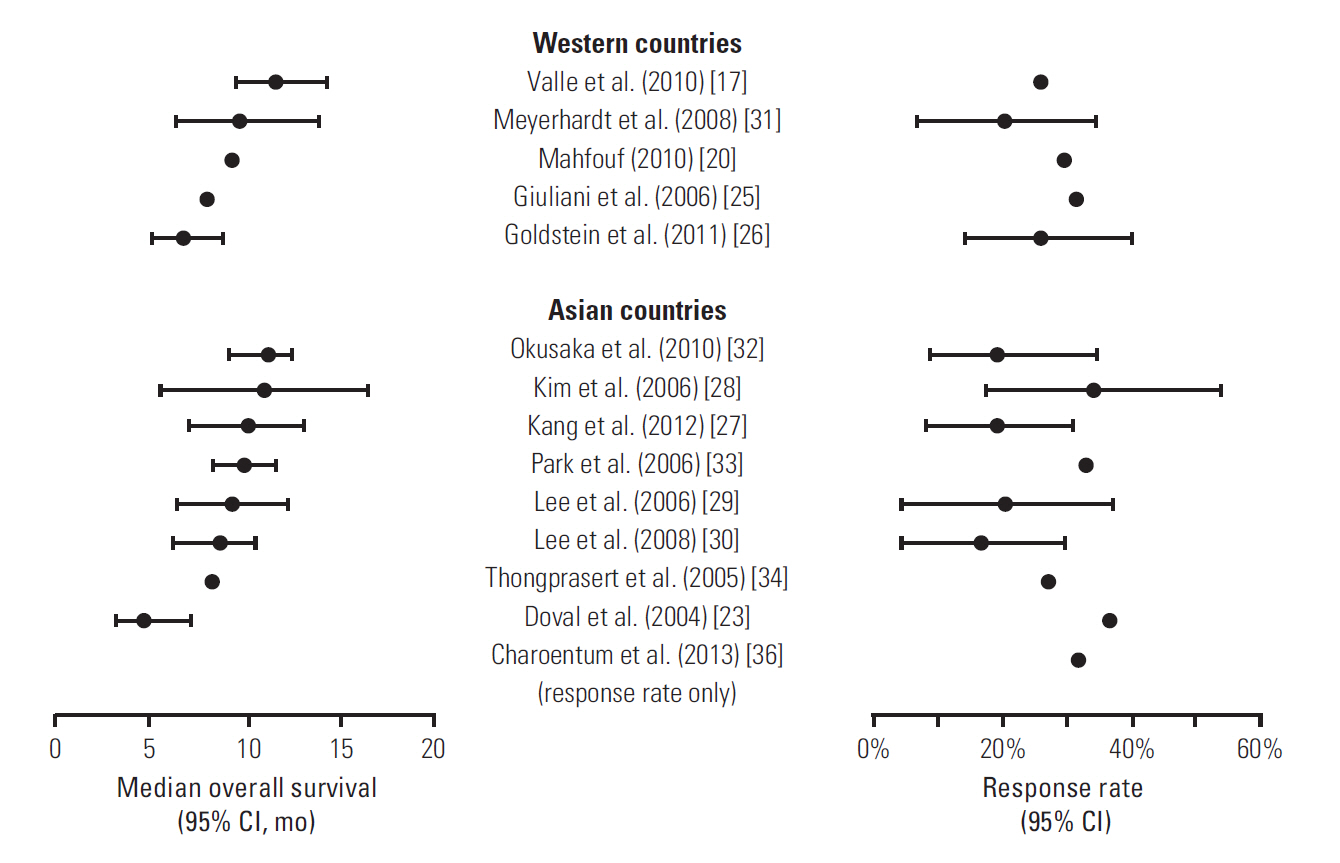
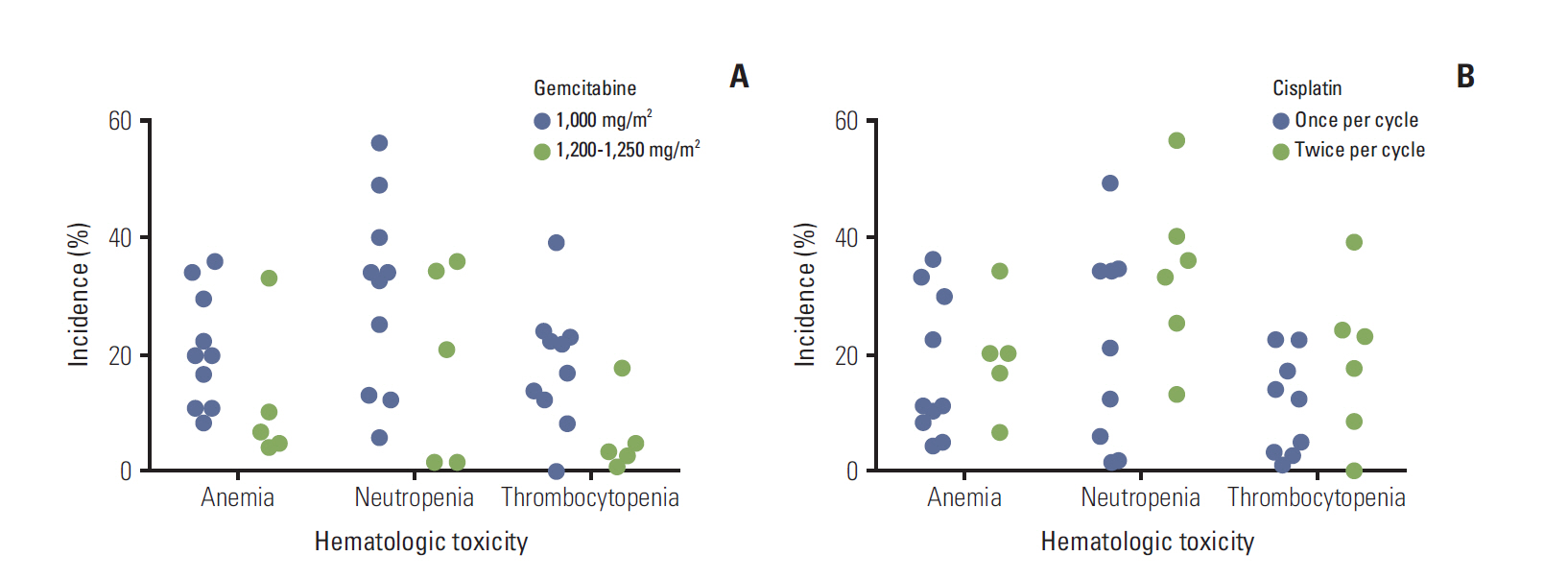
- Evidence suggests that combined gemcitabine-cisplatin chemotherapy extends survival in patients with advanced biliary tract cancer (BTC). We conducted a systematic review in order to collate this evidence and assess whether gemcitabine-cisplatin efficacy is influenced by primary tumor site, disease stage, or geographic region, and whether associated toxicities are related to regimen. MEDLINE (1946-search date), EMBASE (1966-search date), ClinicalTrials. gov (2008-search date), and abstracts from major oncology conferences (2009- search date) were searched (5 Dec 2013) using terms for BTC, gemcitabine, and cisplatin. All study types reporting efficacy (survival, response rates) or safety (toxicities) outcomes of gemcitabine-cisplatin in BTC were eligible for inclusion; efficacy data were extracted from prospective studies only. Evidence retrieved from one meta-analysis (abstract), four randomized controlled trials, 12 nonrandomized prospective studies, and three retrospective studies supported the efficacy and safety of gemcitabine-cisplatin for BTC. Median overall survival ranged from 4.6 to 11.7 months, and response rate ranged from 17.1% to 36.6%. Toxicities were generally acceptable and manageable. Heterogeneity in study designs and data collected prevented formal meta-analysis, however exploratory assessments suggested that efficacy did not vary with primary tumor site (gallbladder vs. others), disease stage (metastatic vs. locally advanced), or geographic origin (Asia vs. other). Incidence of grade 3/4 toxicities was not related to gemcitabine dose or cisplatin frequency. Despite individual variation in study designs, the evidence presented suggests that gemcitabine-cisplatin is effective in patients from a diverse range of countries and with heterogeneous disease characteristics. No substantial differences in toxicity were observed among the different dosing schedules of gemcitabine and cisplatin.
MeSH Terms
Figure
Cited by 1 articles
-
CA19-9 or CEA Decline after the First Cycle of Treatment Predicts Survival in Advanced Biliary Tract Cancer Patients Treated with S-1 and Cisplatin Chemotherapy
Dae-Won Lee, Seock-Ah Im, Yu Jung Kim, Yaewon Yang, Jiyoung Rhee, Im Il Na, Kyung-Hun Lee, Tae-Yong Kim, Sae-Won Han, In Sil Choi, Do-Youn Oh, Jee Hyun Kim, Tae-You Kim, Yung-Jue Bang
Cancer Res Treat. 2017;49(3):807-815. doi: 10.4143/crt.2016.326.
Reference
-
References
1. Noel MS, Hezel AF. New and emerging treatment options for biliary tract cancer. Onco Targets Ther. 2013; 6:1545–52.2. Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med. 2013; 28:515–24.
Article3. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20:146–59.
Article4. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014; 6:99–109.5. Leonard GD, O'Reilly EM. Biliary tract cancers: current concepts and controversies. Expert Opin Pharmacother. 2005; 6:211–23.
Article6. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008; 13:415–23.
Article7. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7:593–600.
Article8. Seufferlein T, Bachet JB, Van Cutsem E, Rougier P; ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23 Suppl 7:vii33–40.
Article9. Gemzar (gemcitabine for injection) [prescribing information]. Indianapolis: Eli Lilly and Company;2013.10. Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabinebased combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012; 18:4944–58.
Article11. Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010; 11:1142–8.
Article12. Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012; 13:181–8.
Article13. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010; 28:4581–6.
Article14. Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010; 11:48–54.
Article15. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007; 96:896–902.
Article16. Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study. The UK ABC-01 Study. Br J Cancer. 2009; 101:621–7.17. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273.
Article18. Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007; 37:843–51.
Article19. Park I, Lee JL, Ryu MH, Kim TW, Lee SS, Park DH, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer. 2009; 115:4148–55.
Article20. Mahfouf H. Chemotherapy using gemcitabine (G) plus cisplatin (C) in locally in stage III and IV gallbladder and biliary tract. J Clin Oncol. 2010; 28 Suppl:e14654.21. Singh D, Kumar S, Sonkar AA, Jaiswal S, Mishra A. Role of low dose prolonged infusion of gemcitabine and cisplatin in inoperable carcinoma gallbladder: a prospective pilot study. Ann Oncol. 2011; 22:v83.22. Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S. Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol. 2007; 13:2852–4.
Article23. Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004; 90:1516–20.
Article24. Eckmann KR, Patel DK, Landgraf A, Slade JH, Lin E, Kaur H, et al. Chemotherapy outcomes for the treatment of unresectable intrahepatic and hilar cholangiocarcinoma: a retrospective analysis. Gastrointest Cancer Res. 2011; 4:155–60.25. Giuliani F, Gebbia V, Maiello E, Borsellino N, Bajardi E, Colucci G, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol. 2006; 17 Suppl 7:vii73–7.
Article26. Goldstein D, Gainford MC, Brown C, Tebbutt N, Ackland SP, van Hazel G, et al. Fixed-dose-rate gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2011; 67:519–25.
Article27. Kang MJ, Lee JL, Kim TW, Lee SS, Ahn S, Park DH, et al. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol. 2012; 51:860–6.
Article28. Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006; 106:1339–46.
Article29. Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol. 2006; 29:127–31.
Article30. Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008; 61:47–52.
Article31. Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008; 53:564–70.
Article32. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010; 103:469–74.
Article33. Park BK, Kim YJ, Park JY, Bang S, Park SW, Chung JB, et al. Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. J Gastroenterol Hepatol. 2006; 21:999–1003.
Article34. Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005; 16:279–81.
Article35. Wu CE, Hsu HC, Shen WC, Lin YC, Wang HM, Chang JW, et al. Chemotherapy with gemcitabine plus cisplatin in patients with advanced biliary tract carcinoma at Chang Gung Memorial Hospital: a retrospective analysis. Chang Gung Med J. 2012; 35:420–7.36. Charoentum C, Chotirosniramit A, Chitapanarux T, Sirivanichai C, Lertprasertsuke N, Chewaskulyong B, et al. Attenuated dose of gemcitabine with cisplatin in patients (pts) with unresectable or metastatic biliary tract cancer (ABC): results of single Thai institution. J Clin Oncol. 2013; 31 Suppl 4:265.
Article37. Mizuno N, Valle JW, Furuse J, Jitlal M, Beare S, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomized trials. J Clin Oncol. 2013; 31 Suppl:4120.
Article38. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014; 25:391–8.
Article39. Thatikonda C, Miller A, Iyer R. Meta analysis of oxaliplatinbased combination chemotherapy for advanced gallbladder and bile duct cancers (AGBC). Am J Gastroenterol. 2010; 105:S72.
Article40. Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2006; 5:110–4.41. Williams KJ, Picus J, Trinkhaus K, Fournier CC, Suresh R, James JS, et al. Gemcitabine with carboplatin for advanced biliary tract cancers: a phase II single institution study. HPB (Oxford). 2010; 12:418–26.
Article42. Gallardo J, Rubio B, Villanueva L, Barajas O. Gallbladder cancer, a different disease that needs individual trials. J Clin Oncol. 2005; 23:7753–4.
Article43. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60:1268–89.
Article44. Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24:3946–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Chemotherapy for Biliary Tract Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Comparison of the Efficacy between Gemcitabine-Cisplatin and Capecitabine-Cisplatin Combination Chemotherapy for Advanced Biliary Tract Cancer
- Durvalumab Plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer (TOPAZ-1)