Cancer Res Treat.
2015 Apr;47(2):259-265. 10.4143/crt.2013.230.
Comparison of the Efficacy between Gemcitabine-Cisplatin and Capecitabine-Cisplatin Combination Chemotherapy for Advanced Biliary Tract Cancer
- Affiliations
-
- 1Department of Internal Medicine Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. angelamd@catholic.ac.kr
- 2Department of Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Hepato-Biliary-Pancreatic Cancer Center, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Cancer Research Institute, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2132804
- DOI: http://doi.org/10.4143/crt.2013.230
Abstract
- PURPOSE
Gemcitabine-cisplatin combination chemotherapy has been regarded as standard regimen for advanced or metastatic biliary tract cancer (BTC), based on the ABC-02 trial. To date, however, no studies have compared the efficacies of gemcitabine-platinum and fluoropyrimidine- platinum combination chemotherapy, even though fluoropyrimidine has been widely used as a backbone agent for gastrointestinal cancer. This study compared the efficacy and toxicities of gemcitabine-cisplatin (GP) and capecitabine-cisplatin (XP) combination chemotherapy for treatment of advanced BTC.
MATERIALS AND METHODS
We examined 49 patients treated with GP and 44 patients treated with XP from October 2009 to July 2012. All patients had unresectable BTC. The GP regimen comprised gemcitabine (1,000 mg/m2, intravenously [IV], days 1 and 8) and cisplatin (75 mg/m2, IV, day 1). The XP regimen comprised capecitabine (1,250 mg/m2 twice a day, peroral, days 1-14) and cisplatin (60 mg/m2, IV, day 1, every three weeks). We analyzed the response rate (RR), time to progression (TTP), overall survival (OS), and toxicity.
RESULTS
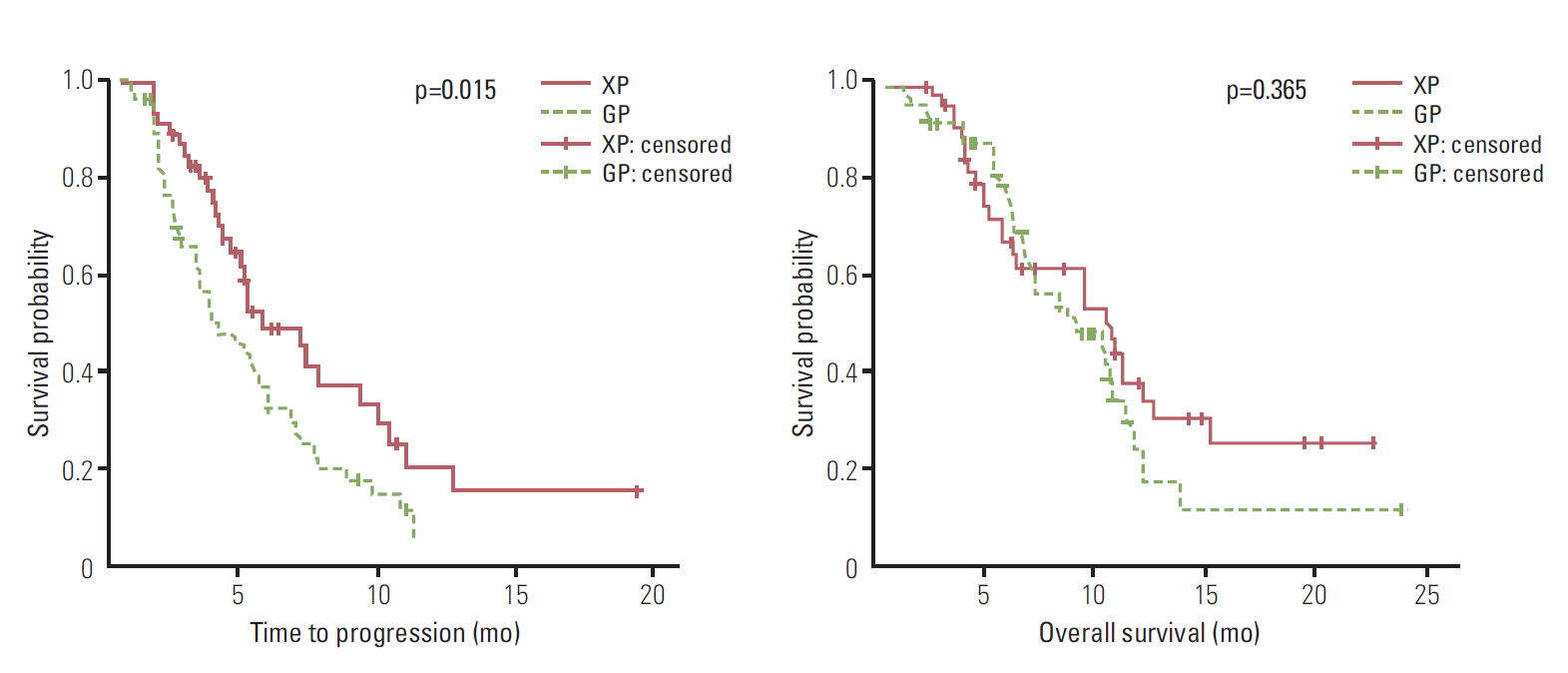
The RRs were 27.3% and 6.1% in the XP and GP arms, respectively. XP resulted in longer TTP (5.2 months vs. 3.6 months, p=0.016), but OS was not statistically different (10.7 months vs. 8.6 months, p=0.365). Both regimens resulted in grade 3-4 hematologic toxicities, but febrile neutropenia was not noted. Grade 3-4 asthenia, stomatitis, and hand-foot syndrome occurred more frequently in the XP arm.
CONCLUSION
XP resulted in a superior TTP and RR compared to GP for treatment of advanced BTC, with comparable toxicity. Conduct of prospective large, randomized trials to evaluate the possibility of XP as another standard therapy is warranted.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
CA19-9 or CEA Decline after the First Cycle of Treatment Predicts Survival in Advanced Biliary Tract Cancer Patients Treated with S-1 and Cisplatin Chemotherapy
Dae-Won Lee, Seock-Ah Im, Yu Jung Kim, Yaewon Yang, Jiyoung Rhee, Im Il Na, Kyung-Hun Lee, Tae-Yong Kim, Sae-Won Han, In Sil Choi, Do-Youn Oh, Jee Hyun Kim, Tae-You Kim, Yung-Jue Bang
Cancer Res Treat. 2017;49(3):807-815. doi: 10.4143/crt.2016.326.
Reference
-
References
1. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007; 96:896–902.
Article2. Jang JS, Lim HY, Hwang IG, Song HS, Yoo N, Yoon S, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group phase II trial. Cancer Chemother Pharmacol. 2010; 65:641–7.
Article3. Valle JW. Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol. 2010; 21 Suppl 7:vii345–8.
Article4. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009; 20:146–59.
Article5. Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Seo HG, et al. Prediction of cancer incidence and mortality in Korea, 2012. Cancer Res Treat. 2012; 44:25–31.
Article6. Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012; 13:181–8.
Article7. Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008; 13:415–23.
Article8. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006; 118:1591–602.
Article9. Hong YS, Lee J, Lee SC, Hwang IG, Choi SH, Heo JS, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother Pharmacol. 2007; 60:321–8.
Article10. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996; 7:593–600.
Article11. Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, et al. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998; 9:653–6.
Article12. Ellis PA, Norman A, Hill A, O'Brien ME, Nicolson M, Hickish T, et al. Epirubicin, cisplatin and infusional 5-fluorouracil (5-FU) (ECF) in hepatobiliary tumours. Eur J Cancer. 1995; 31:1594–8.
Article13. Dingle BH, Rumble RB, Brouwers MC; Cancer Care Ontario's Program in Evidence-Based Care's Gastrointestinal Cancer Disease Site Group. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005; 19:711–6.
Article14. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article15. Kim TW, Chang HM, Kang HJ, Lee JR, Ryu MH, Ahn JH, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann Oncol. 2003; 14:1115–20.
Article16. Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006; 106:1339–46.
Article17. Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008; 8:374.
Article18. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–73.
Article19. Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012; 23:2479–516.
Article20. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010; 103:469–74.
Article21. Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008; 61:47–52.
Article22. Giuliani F, Gebbia V, Maiello E, Borsellino N, Bajardi E, Colucci G, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol. 2006; 17 Suppl 7:vii73–7.
Article23. Andre T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004; 15:1339–43.24. Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008; 98:309–15.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy for Biliary Tract Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Real-World Outcomes of Gemcitabine, Cisplatin, and Nab-Paclitaxel Chemotherapy Regimen for Advanced Biliary Tract Cancer: A Propensity Score-Matched Analysis
- Efficacy and Toxicity of Gemcitabine Based Chemotherapy for Advanced Urothelial Cancer