Korean J Urol.
2007 Oct;48(10):997-1003. 10.4111/kju.2007.48.10.997.
Utility of Smo as a Prognostic Marker for Human Bladder Tumors
- Affiliations
-
- 1Department of Urology, College of Medicine, Chungbuk National University, Cheongju, Korea. wjkim@chungbuk. ac.kr
- KMID: 2139729
- DOI: http://doi.org/10.4111/kju.2007.48.10.997
Abstract
-
PURPOSE: Smoothened(Smo) encodes a 1,024 amino acid transmembrane protein that acts as a transducer of the hedgehog(Hh) signal and maps to 7q31-q32 in humans. In the absence of Hh, Patched(Ptc) prevents Smo from signaling. When M-Hh-N binds to Ptc, however, Smo is free to upregulate downstream genes in the network. Activating mutations in Smo have been identified in sporadic basal cell carcinomas. This study was performed to evaluate the significance of Smo expression in humanzbladder cancer.
MATERIALS AND METHODS
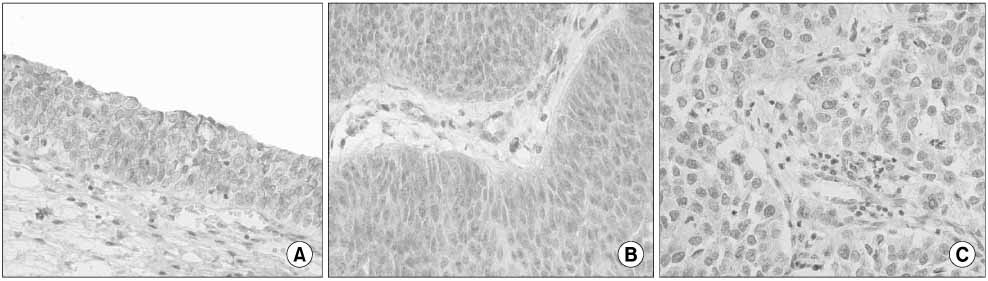
Tumor tissues were obtained from 140 patients with bladder cancer and normal bladder mucosa were acquired from 17 patients without bladder cancer as controls. Smo expression was assessed from paraffin sections of tissues using immunohistochemistry and graded on a scale of 0-12 according to the intensity and rate of staining. Differences of Smo expression between the bladder tumors and normal mucosa were compared. The relationships between their expression and the pathological or clinical characteristics such as tumor stage, grade, recurrence, and progression were also analyzed.
RESULTS
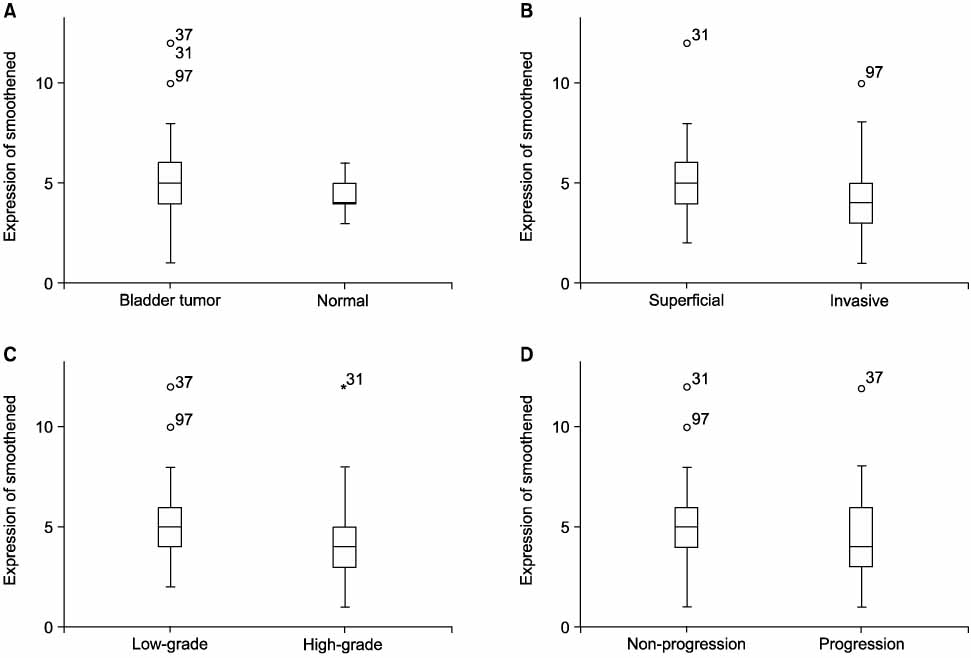
There was no difference in the Smo expression in comparisons between the bladder cancers and the normal tissues(4.96+/-1.92 vs.4.52+/-0.87, p=0.111). Superficial bladder tumors had a higher Smo expression compared with normal tissues(0.005). Smo expression in the superficial and low-grade bladder tumors were higher than in the invasive and high-grade bladder tumors(p=0.002 and 0.001, respectively). The progression status was correlated with Smo expression but not the recurrence status(p=0.041 and 0.357, respectively). However, the Smo expression levels were not associated with the overall survival of patients(p=0.406).
CONCLUSIONS
The results of this study showed that the enhanced expression of Smo was correlated with superficial, low-grade bladder cancer and tumors without progression. These results suggest that Smo is closely correlated with the differentiation and progression of bladder cancer and may, therefore, be useful as a prognostic marker for bladder cancer in the clinical setting.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Significance of the Expression of Gli2 and Gli3 in Bladder Cancer
Kwang-Hee Han, Yunbyung Chae, Pildu Jeong, Yong-June Kim, Seok-Joong Yun, Sang-Cheol Lee, Wun-Jae Kim
Korean J Urol. 2008;49(8):696-702. doi: 10.4111/kju.2008.49.8.696.
Reference
-
1. Hudson MA, Catalona WJ. Gillenwater JY, Grayhack JT, editors. Urothelial tumors of the bladder, upper tracts and prostate. Adult and pediatric urology. 1996. 3rd ed. St. Louis: Mosby;1379–1464.2. Messing EM. Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA, editors. Urothelial tumors of the bladder. Campbell-Walsh urology. 2006. 9th ed. Pennsylvania: Saunders;2426–2427.3. Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001. 15:3059–3087.4. Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the Hedgehog receptor. Nature. 1996. 384:176–179.5. Hooper JE, Scott MP. The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell. 1989. 59:751–765.6. Xie J, Murone M, Luoh S, Ryan A, Gu Q, Zhang C, et al. Activating smoothened mutations in sporadic basal-cell carcinomas. Nature. 1998. 391:90–92.7. Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, et al. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998. 58:1798–1803.8. Hallahan AR, Pritchard JI, Hansen S, Benson M, Stoeck J, Hatton BA, et al. The Smo A1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 2004. 64:7794–7800.9. Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, et al. The tumour-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996. 384:129–134.10. Chen Y, Struhl G. Dual roles for patched in sequestering and transducing Hedgehog. Cell. 1996. 87:553–563.11. Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell. 1996. 86:221–232.12. Kim SH, Kook MC, Shin YK, Park SH, Song HG. Evaluation of antigen retrieval buffer systems. J Mol Histol. 2004. 35:409–416.13. Kim SH, Shin YK, Lee KM, Park SH, Song HG. An improved protocol of biotinylated tyramine-based immunohistochemistry minimizing nonspecific background staining. J Histochem Cytochem. 2003. 51:129–132.14. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–241.15. Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001. 2:172–180.16. Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003. 6:21–27.17. Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003. 39:937–950.18. Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004. 432:324–331.19. Watikins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and small-cell lung cancer. Nature. 2003. 422:313–317.20. Marigo V, Davey RA, Zuo Y, Cunningham JM, Tabin CJ. Biochemical evidence that patched is the hedgehog receptor. Nature. 1996. 384:176–179.21. Motoyama J, Takabatake T, Takeshima K, Hui C. Ptch2, a second mouse patched gene, is co-expressed with sonic hedgehog. Nat Genet. 1998. 18:104–106.22. Carpenter D, Stone DM, Brush J, Ryan A, Armanini M, Frantz G, et al. Characterization of two patched receptors for the vertebrate hedgehog protein family. Proc Natl Acad Sci USA. 1998. 95:13630–13634.23. Zaphiropoulos PG, Unden AB, Rahnama F, Hollingsworth RE, Toftgard R. PTCH2, a novel human patched gene, undergoing alternative splicing and up-regulated in basal cell carcinomas. Cancer Res. 1999. 59:787–792.24. Smyth I, Narang MA, Evans T, Heimann C, Nakamura Y, Chenevix-Trench G, et al. Isolation and characterization of human patched 2 (PTCH2), a putative tumour suppressor gene in basal cell carcinomas and medulloblastoma on chromosome 1p32. Hum Mol Genet. 1999. 8:291–297.25. Hynes M, Ye W, Wang K, Stone D, Murone M, de Sauvage F, et al. The seven-transmembrane receptor smoothened cell-autonomously induces multiple ventral cell types. Nat Neurosci. 2000. 3:41–46.26. Thayer SP, di Magliano MP, Heiser PW, Nielson CM, Roberts DJ, Lauwers GY, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003. 425:851–856.27. Yoshizaki A, Nakayama T, Natio S, Wen CY, Sekine I. Expressions of sonic hedgehog, patched, smoothened and Gli-1 in human intestinal stromal tumors and their correlation with prognosis. World J Gastroenterol. 2006. 35:5687–5691.28. Huang S, He J, Zhang X, Bian Y, Yang L, Xie G, et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis. 2006. 27:1334–1340.29. Al-Sukhun S, Hussain M. Molecular biology of transitional cell carcinoma. Crit Rev Oncol Hematol. 2003. 47:181–193.30. Ogden SK, Ascano M Jr, Stegman MA, Robbins DJ. Regulation of Hedgehog signaling: a complex story. Biochem Pharmacol. 2004. 67:805–814.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chromosomal changes in transitional cell carcinoma of the bladder
- Prognostic Value of Expression of c-erbB-2 in Urinary Bladder Cancer
- Can we use methylation markers as diagnostic and prognostic indicators for bladder cancer?
- The prognostic factors influencing the recurrence rate of superficial bladder cancer
- Significance of Telomerase Activity as the Prognostic Factor of Human Bladder Cancer