J Korean Med Assoc.
2015 Jun;58(6):516-522. 10.5124/jkma.2015.58.6.516.
Advances in magnetic resonance technique for tumor imaging
- Affiliations
-
- 1Department of Radiology, Hanyang University Guri Hospital, Guri, Korea. dwpark@hanyang.ac.kr
- KMID: 2137558
- DOI: http://doi.org/10.5124/jkma.2015.58.6.516
Abstract
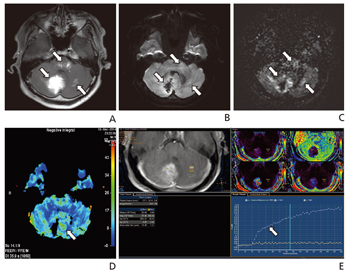
- The development of advanced magnetic resonance (MR) imaging techniques for tumors has not only lead to improved diagnostic accuracy, but has also assisted with tumor staging, surgical planning, and postoperative follow-up study. Recently introduced and/or clinically used MR imaging techniques for tumors, including chemical exchange saturation transfer imaging, molecular imaging, magnetic resonance spectroscopy, perfusion, and blood flow suppression techniques, could improve diagnostic accuracy and provide useful information to guide the management of tumors. It is essential to properly obtain and evaluate advanced MR images for tumors, depending on the specific characteristics of each tumor.
Keyword
Figure
Cited by 1 articles
-
Recent trends in radiology
Eun-Young Kang
J Korean Med Assoc. 2015;58(6):499-501. doi: 10.5124/jkma.2015.58.6.499.
Reference
-
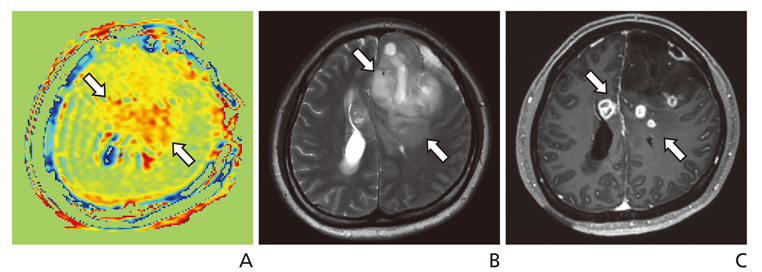
1. Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, Messina SA, Eberhart CG, Pomper MG, Laterra J, Barker PB, van Zijl PC, Blakeley JO. Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging. 2013; 38:1119–1128.
Article2. Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Suzuki Y, Suzuki SO, Iwaki T, Hata N, Mizoguchi M, Yoshimoto K, Sagiyama K, Takahashi M, Honda H. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol. 2014; 16:441–448.
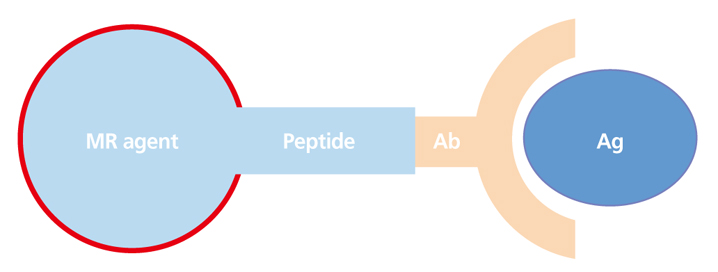
Article3. Song HT, Suh JS. Cancer-targeted MR molecular imaging. J Korean Med Assoc. 2009; 52:121–124.
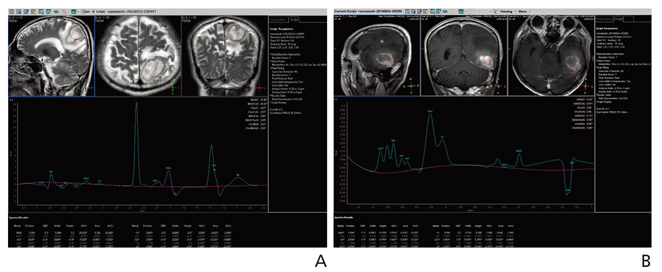
Article4. Caivano R, Lotumolo A, Rabasco P, Zandolino A, D'Antuono F, Villonio A, Lancellotti MI, Macarini L, Cammarota A. 3 Tesla magnetic resonance spectroscopy: cerebral gliomas vs metastatic brain tumors Our experience and review of the literature. Int J Neurosci. 2013; 123:537–543.
Article5. Bulik M, Jancalek R, Vanicek J, Skoch A, Mechl M. Potential of MR spectroscopy for assessment of glioma grading. Clin Neurol Neurosurg. 2013; 115:146–153.
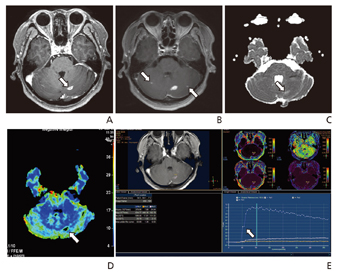
Article6. Jahng GH, Kim HS, Kim SM, Ryu CW. Principles and technical aspects of perfusion magnetic resonance imaging. J Korean Soc Magn Reson Med. 2011; 15:91–101.
Article7. Kim HG, Jahng GH, Oh CH. Simulations of perfusion signals of pulsed arterial spin labeling MRI. J Korean Soc Magn Reson Med. 2011; 15:191–199.
Article8. Cho SK, Na DG, Ryoo JW, Roh HG, Moon CH, Byun HS, Kim JH. Perfusion MR imaging: clinical utility for the differential diagnosis of various brain tumors. Korean J Radiol. 2002; 3:171–179.
Article9. Choi CG, Jung AK, Kim JH, Kang SH, Lee HK. Perfusion MR imaging of cerebral gliomas: comparison with histologic tumor grade. J Korean Soc Magn Reson Med. 2001; 5:130–137.10. Koo JH, Yoon YC, Kim JH. Diffusion-weighted and dyna-mic contrast-enhanced MRI of metastatic bone tumors: correlation of the apparent diffusion coefficient, K(trans) and ve values. J Korean Soc Magn Reson Med. 2014; 18:25–33.
Article11. Kim YT, Chung TW, Yoon W, Kang HK. The usefulness of diffusion-tensor MR imaging (DTI) in the grading of gliomas. J Korean Radiol Soc. 2008; 58:333–339.
Article12. Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to Dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011; 21:1233–1242.
Article13. Jeong B, Choi DS, Shin HS, Choi HY, Park MJ, Jeon KN, Na JB, Chung SH. T1-weighted FLAIR MR imaging for the evaluation of enhancing brain tumors: comparison with spin echo imaging. J Korean Soc Magn Reson Med. 2014; 18:151–156.
Article14. Nagao E, Yoshiura T, Hiwatashi A, Obara M, Yamashita K, Kamano H, Takayama Y, Kobayashi K, Honda H. 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. AJNR Am J Neuroradiol. 2011; 32:664–670.
Article15. Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging. 2010; 31:1256–1263.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional Magnetic Resonance Imaging of the Brain: Principle and Practical Application
- High field strength magnetic resonance imaging of cardiovascular diseases
- Advanced Magnetic Resonance Imaging for Pediatric Brain Tumors: Current Imaging Techniques and Interpretation Algorithms
- Ultrafast Magnetic Resonance Imaging: Echo Planar Imaging and Spiral Scan Imaging
- Modern Brain Tumor Imaging