J Clin Neurol.
2010 Mar;6(1):27-32. 10.3988/jcn.2010.6.1.27.
Frovatriptan is Effective and Well Tolerated in Korean Migraineurs: A Double-Blind, Randomized, Placebo-Controlled Trial
- Affiliations
-
- 1Department of Neurology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Neurology, Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Uijeongbu St. Mary's Hospital, The Catholic Univeristy of Korea College of Medicine, Uijeongbu, Korea.
- 4Department of Neurology, Korea University College of Medicine, Seoul, Korea.
- 5Department of Neurology, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 6Department of Neurology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea.
- 7Department of Neurology, Pusan Paik Hospital, Inje University College of Medicine, Busan, Korea.
- 8Department of Neurology, Catholic University of Daegu School of Medicine, Daegu, Korea.
- 9Department of Statistics, Seo Kyeong University, Seoul, Korea.
- 10Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sukwon@amc.seoul.kr
- KMID: 2135478
- DOI: http://doi.org/10.3988/jcn.2010.6.1.27
Abstract
- BACKGROUND AND PURPOSE
Frovatriptan is a selective 5-HT1B/1D agonist with a long duration of action and a low incidence of side effects. Although several placebo-controlled trials have documented the clinical efficacy and safety of frovatriptan in adults with migraine, this drug has not previously been studied in Asian including Korean patients.
METHODS
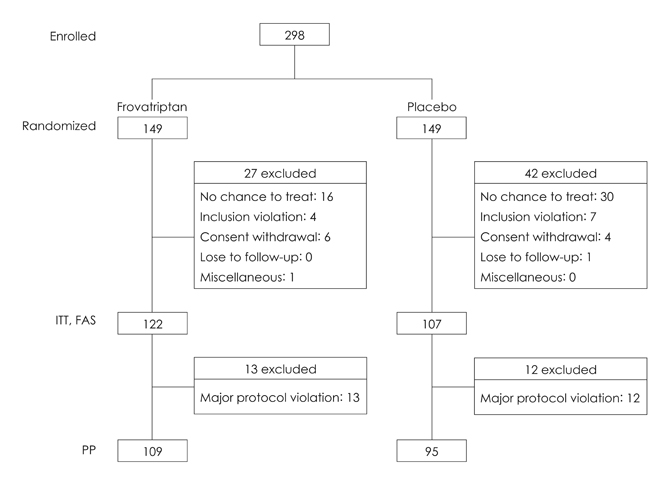
In this double-blind multicenter trial, 229 patients with migraine were randomized to receive frovatriptan 2.5 mg or placebo upon the occurrence of a moderate-to-severe migraine. The primary outcome was the 2-hour headache response rate.
RESULTS
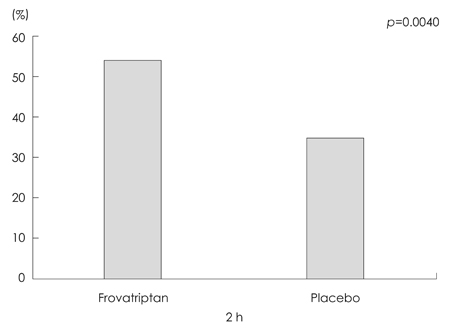
Frovatriptan significantly increased the 2-hour headache response rate compared with placebo (52.9% vs. 34.0%, p=0.004). The headache response rates at 4, 6, and 12 hours were significantly higher in the frovatriptan group than in the placebo group, as was the pain-free rate at 2 hours (19.0% vs. 5.7%, p=0.004), 4 hours (40.7% vs. 23.0%, p=0.006), and 6 hours (56.1% vs. 34.0%, p=0.002). The median time to a headache response was significantly shorter in the frovatriptan group than in the placebo group (2.00 hours vs. 3.50 hours, p<0.001). The use of rescue medications was more common in the placebo group (p=0.005). Chest tightness associated with triptan was infrequent (2.5%), mild, and transient.
CONCLUSIONS
These results demonstrate that 2.5-mg frovatriptan is effective and well tolerated in Korean migraineurs for acute treatment of migraine attacks.
Keyword
MeSH Terms
Figure
Reference
-
1. Ferrari MD, Saxena PR. On serotonin and migraine: a clinical and pharmacological review. Cephalalgia. 1993. 13:151–165.
Article2. Tfelt-Hansen P, De Vries P, Saxena PR. Triptans in migraine: a comparative review of pharmacology, pharmacokinetics and efficacy. Drugs. 2000. 60:1259–1287.3. Brown AM, Parsons AA, Raval P, Porter R, Tilford NS, Gager TL, et al. SB 209509 (VML 251), a potent constrictor of rabbit basilar artery with high affinity and selectivity for human 5-HT1D receptors. Br J Pharmacol. 1996. 119:110P.4. Goldstein J, Keywood C. 251/96/14 Study Group. Frovatriptan for the acute treatment of migraine: a dose-finding study. Headache. 2002. 42:41–48.
Article5. Kang L, Lee ST, Im W, Kim SC, Hun KS, Kim BK, et al. Screening of the A11084G polymorphism and scanning of a mitochondrial genome SNP in Korean migraineurs. J Clin Neurol. 2007. 3:127–132.
Article6. Sakai F, Iwata M, Tashiro K, Itoyama Y, Tsuji S, Fukuuchi Y, et al. Zolmitriptan is effective and well tolerated in Japanese patients with migraine: a dose-response study. Cephalalgia. 2002. 22:376–383.
Article7. Sakai F, Diener HC, Ryan R, Poole P. Eletriptan for the acute treatment of migraine: results of bridging a Japanese study to Western clinical trials. Curr Med Res Opin. 2004. 20:269–277.
Article8. Shimazawa R, Ando Y, Hidaka S, Saito K, Toyoshima S, Kobayashi F. Development of triptans in Japan: bridging strategy based on the ICH-E5 guideline. J Health Sci. 2006. 52:443–449.
Article9. Yates RA, Tateno M, Nairn K, Ikegami A, Dane A, Kemp J. The pharmacokinetics of the antimigraine compound zolmitriptan in Japanese and Caucasian subjects. Eur J Clin Pharmacol. 2002. 58:247–252.
Article10. Rapoport A, Ryan R, Goldstein J, Keywood C. Dose range-finding studies with frovatriptan in the acute treatment of migraine. Headache. 2002. 42:Suppl 2. S74–S83.
Article11. Ryan R, Géraud G, Goldstein J, Cady R, Keywood C. Clinical efficacy of frovatriptan: placebo-controlled studies. Headache. 2002. 42:Suppl 2. S84–S92.
Article12. Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004. 63:261–269.
Article13. Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 2nd edition. Cephalalgia. 2004. 24:Suppl 1. 1–160.14. Protocol No. 251/96/07 Clinical Study Report. 2002. Vernalis.15. Lee SB, Kim YI, Choi YB, Chung SW, Yang DW, Lee KS, et al. Double-blind placebo-controlled randomized clinical trial of zolmitriptan in acute treatment of migraine. J Korean Neurol Assoc. 2001. 19:29–35.16. Havanka H, Dahlöf C, Pop PH, Diener HC, Winter P, Whitehouse H, et al. Naratriptan S2WB2004 Study Group. Efficacy of naratriptan tablets in the acute treatment of migraine: a dose-ranging study. Clin Ther. 2000. 22:970–980.
Article17. Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5-HT(1B/1D) agonists) in acute migraine treatment: a meta-analysis of 53 trials. Lancet. 2001. 358:1668–1675.
Article18. Jo KD, Lee MC. Oral sumatriptan for acute treatment of migraine A single-blind placebo-controlled study. J Korean Neurol Assoc. 1995. 13:77–83.19. Noh YW, Lee TG, Park KH, Kim SM, Chung KC. A double-blinded, placebo-controlled, multicenter cross-over study of 2.5mg naratriptan in acute migraineurs. Korean J Headache. 2001. 2:53–60.20. Pfaffenrath V, Cunin G, Sjonell G, Prendergast S. Efficacy and safety of sumatriptan tablets (25 mg, 50 mg, and 100 mg) in the acute treatment of migraine: defining the optimum doses of oral sumatriptan. Headache. 1998. 38:184–190.
Article21. Rapoport AM, Ramadan NM, Adelman JU, Mathew NT, Elkind AH, Kudrow DB, et al. The 017 Clinical Trial Study Group. Optimizing the dose of zolmitriptan (Zomig, 311C 90) for the acute treatment of migraine. A multicenter, double-blind, placebo-controlled, dose range-finding study. Neurology. 1997. 49:1210–1218.
Article22. Dahlöf C. Safety and tolerability of the novel 5-HT 1B/1D agonist rizatriptan in migraineurs. Headache. 1999. 39:Suppl 1. S16–S18.
Article23. Wang SJ, Fuh JL, Wu ZA. Intranasal sumatriptan study with high placebo response in Taiwanese patients with migraine. J Chin Med Assoc. 2007. 70:39–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of cetirizine in dogs with chronic atopic dermatitis: a randomized, double blind, placebo-controlled trial
- Adjuvant Sertraline Treallnent for Chronic Schizophrenia :A Randomized, Double Blind, Placeho-Controlled Study
- Treatment of Knee Osteoarthritis with Ketoprofen(Ketotop): A Double-blind Placebo-controlled Randomized Trial
- Double-Blind Placebo-Controlled Randomized Clinical Trial of Zolmitriptan in Acute Treatment of Migraine
- Double-blind, Parallel-group, Placebo-controlled Multi-center Clinical Trial for Evaluating the Efficacy and Safety of Phentermine Hydrochloride in Obese Patients