J Cardiovasc Ultrasound.
2009 Jun;17(2):60-69. 10.4250/jcu.2009.17.2.60.
Effects of Granulocyte-Colony Stimulating Factor and Bone Marrow Mononuclear Cells on Cardiac Function and Remodeling in the Porcine Reperfused Myocardial Infarction Model
- Affiliations
-
- 1Department of Cardiology, Sejong General Hospital, Bucheon, Korea.
- 2Department of Cardiology, Cardiovascular Center, Korea University Anam Hospital, Seoul, Korea. wjshimmd@unitel.co.kr
- KMID: 2135446
- DOI: http://doi.org/10.4250/jcu.2009.17.2.60
Abstract
- BACKGROUND
Granulocyte stimulating factor (G-CSF) and bone marrow mononuclear cells (BM-MNC) were reported to improve cardiac function after myocardial infarction (MI). This study was to examine their combined beneficial effects and mechanisms of actions in reperfused MI, which have not been verified yet.
METHODS
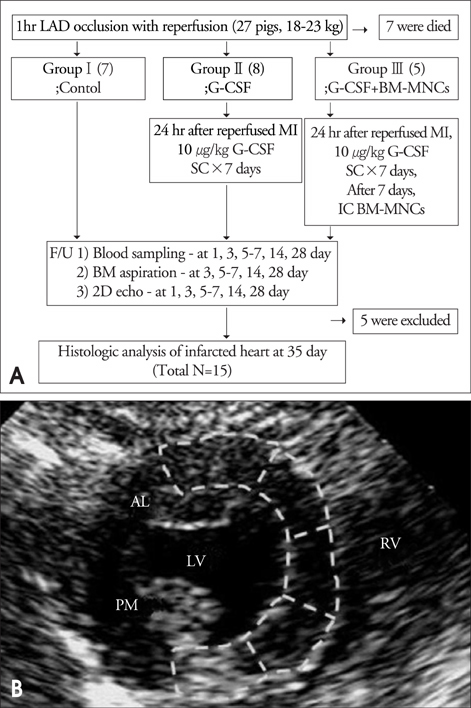
Fifteen pigs were divided into 3 groups after a 1-hour balloon occlusion and reperfusion of the left anterior descending coronary artery. G1 (n=5) was a control, G2 (n=5) was a G-CSF injected group (10 ug/kg/day, from day1 to day7 after MI), and G3 (n=5) was an autologous intracoronary BM-MNC infused group after G-CSF treatment
RESULTS
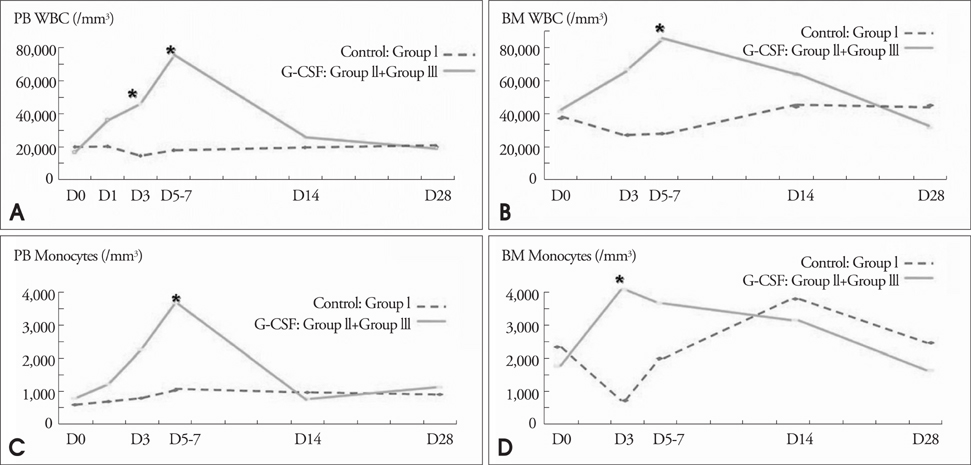
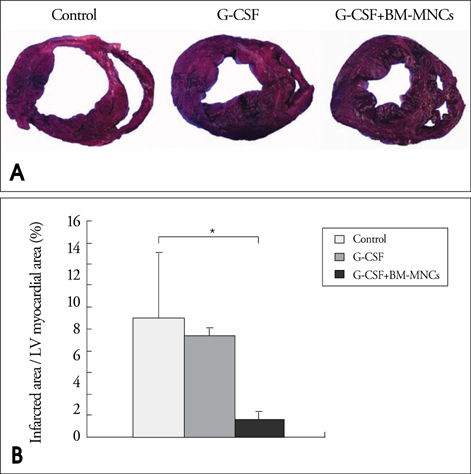
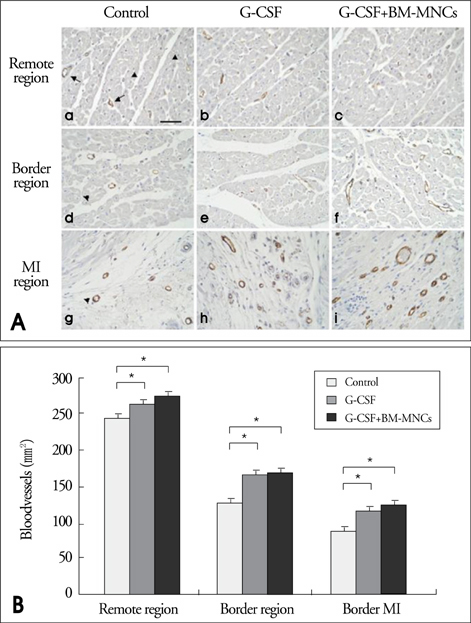
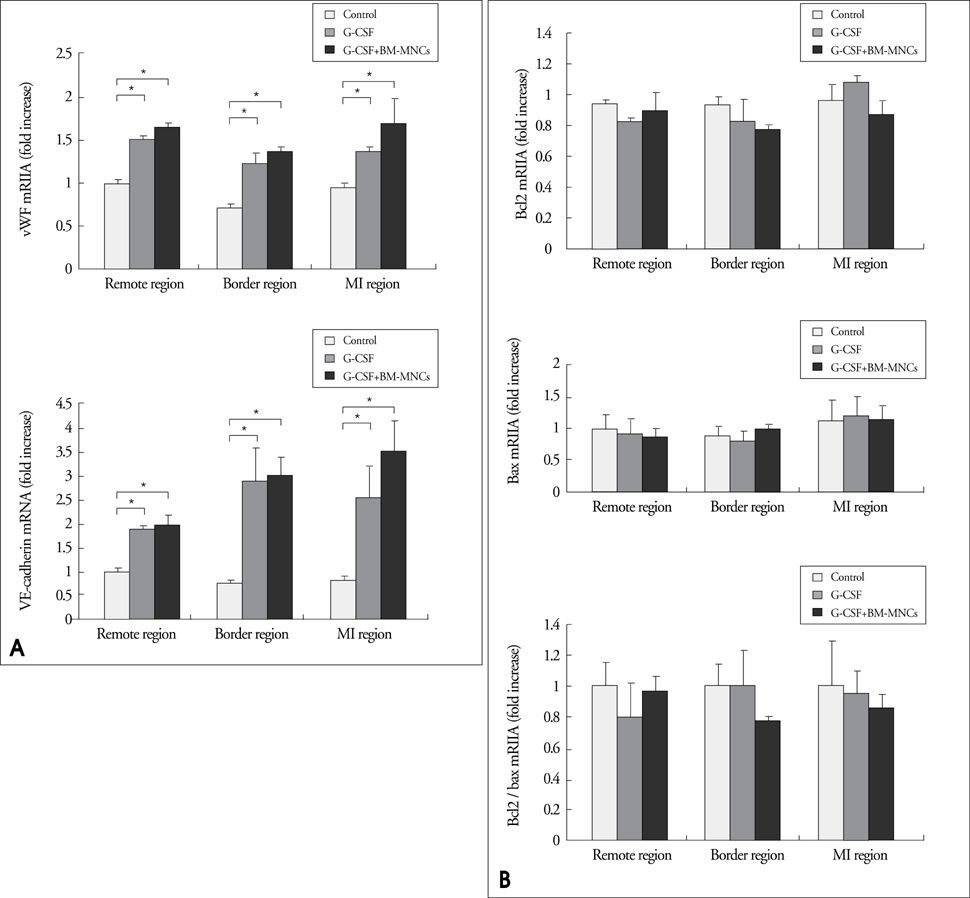
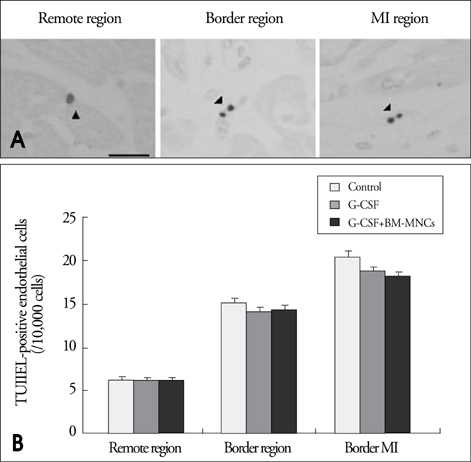
Modified wall motion indices by echocardiography were similar among 3 groups at 24 hours after MI. However, they improved significantly in G2 and G3 at 35days after MI (p<0.05). The percentage of infarct area/left ventricular myocardial area measured from a triphenyltetrazolium chloride (TTC) stain was lower in G3 than in G1 or G2 (p=0.026). The number of vWF-positive vessels and the expressions of vWF and VE cardherin by RT-PCR were higher in G3 and G2 than in G1 (p<0.05). The number of TUNEL-positive cells and bcl2/bax ratio were not significantly different among 3 groups.
CONCLUSION
This study suggests that intracoronary BM-MNC infusion with G-CSF treatment in reperfused MI reduced infarct size, improved left ventricular function and prevented ventricular remodeling.
Keyword
MeSH Terms
Figure
Reference
-
1. Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991. 260:H1406–H1414.
Article2. Pfeffer MA, Braunwald E. Ventricular remodeling after myocar-dial infarction. Experimental observations and clinical implications. Circulation. 1990. 81:1161–1172.
Article3. Braunwald E, Kim CB. Late establishment of patency of the infarct-related artery. 1994. London: Saunders.4. Schmermund A, Lerman LO, Ritman EL, Rumberger JA. Cardiac production of angiotensin II and its pharmacologic inhibition: effects on the coronary circulation. Mayo Clin Proc. 1999. 74:503–513.
Article5. Adachi Y, Imagawa J, Suzuki Y, Yogo K, Fukazawa M, Kuromaru O, Saito Y. G-CSF treatment increases side population cell infiltration after myocardial infarction in mice. J Mol Cell Cardiol. 2004. 36:707–710.
Article6. Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004. 428:668–673.
Article7. Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005. 11:305–311.
Article8. Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001. 104:1046–1052.
Article9. Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, Kim YJ, Soo Lee D, Sohn DW, Han KS, Oh BH, Lee MM, Park YB. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: the MAGIC cell randomised clinical trial. Lancet. 2004. 363:751–756.
Article10. Kuethe F, Richartz BM, Kasper C, Sayer HG, Hoeffken K, Werner GS, Figulla HR. Autologous intracoronary mononuclear bone marrow cell transplantation in chronic ischemic cardiomyopathy in humans. Int J Cardiol. 2005. 100:485–491.
Article11. Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004. 428:664–668.
Article12. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.
Article13. Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002. 106:1913–1918.
Article14. Tomita S, Mickle DA, Weisel RD, Jia ZQ, Tumiati LC, Allidina Y, Liu P, Li RK. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002. 123:1132–1140.
Article15. Wojakowski W, Tendera M, Zebzda A, Michalowska A, Majka M, Kucia M, Maslankiewicz K, Wyderka R, Krol M, Ochala A, Kozakiewicz K, Ratajczak MZ. Mobilization of CD34(+), CD117(+), CXCR4(+), c-met(+) stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006. 27:283–289.
Article16. Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004. 364:141–148.
Article17. Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001. 7:430–436.
Article18. Kuethe F, Figulla HR, Herzau M, Voth M, Fritzenwanger M, Opfermann T, Pachmann K, Krack A, Sayer HG, Gottschild D, Werner GS. Treatment with granulocyte colony-stimulating factor for mobilization of bone marrow cells in patients with acute myocardial infarction. Am Heart J. 2005. 150:115.
Article19. Link DC. Mechanisms of granulocyte colony-stimulating factor-induced hematopoietic progenitor-cell mobilization. Semin Hematol. 2000. 37:25–32.
Article20. Ohtsuka M, Takano H, Zou Y, Toko H, Akazawa H, Qin Y, Suzuki M, Hasegawa H, Nakaya H, Komuro I. Cytokine therapy prevents left ventricular remodeling and dysfunction after myocardial infarction through neovascularization. Faseb J. 2004. 18:851–853.
Article21. Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001. 98:10344–10349.
Article22. Iwanaga K, Takano H, Ohtsuka M, Hasegawa H, Zou Y, Qin Y, Odaka K, Hiroshima K, Tadokoro H, Komuro I. Effects of G-CSF on cardiac remodeling after acute myocardial infarction in swine. Biochem Biophys Res Commun. 2004. 325:1353–1359.
Article23. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999. 103:697–705.
Article24. Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999. 100:II247–II256.
Article25. Bayne K. Revised Guide for the Care and Use of Laboratory Animals available. American Physiological Society. Physiologist. 1996. 39:199208–211.26. Avalos BR. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood. 1996. 88:761–777.
Article27. Berliner N, Hsing A, Graubert T, Sigurdsson F, Zain M, Bruno E, Hoffman R. Granulocyte colony-stimulating factor induction of normal human bone marrow progenitors results in neutrophil-specific gene expression. Blood. 1995. 85:799–803.
Article28. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991. 78:2791–2808.
Article29. Fujii K, Ishimaru F, Kozuka T, Matsuo K, Nakase K, Kataoka I, Tabayashi T, Shinagawa K, Ikeda K, Harada M, Tanimoto M. Elevation of serum hepatocyte growth factor during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Br J Haematol. 2004. 124:190–194.
Article30. Ohmi C, Matsuyama H, Tei Y, Yoshihiro S, Shimabukuro T, Ohmoto Y, Naito K. Granulocyte colony-stimulating factor may promote proliferation of human bladder cancer cells mediated by basic fibroblast growth factor. Scand J Urol Nephrol. 2003. 37:286–291.
Article31. Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003. 195:158–167.
Article32. Abbate A, Biondi-Zoccai GG, Baldi A. Pathophysiologic role of myocardial apoptosis in post-infarction left ventricular remodeling. J Cell Physiol. 2002. 193:145–153.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A study of the effects of GM-CSF incubation on long-term human bone marrow cultures
- Current Status of G-CSF Based Stem Cell Therapy for Patients with Myocardial Infarction
- Stem Cell Therapy for Patients with Myocardial Infarction
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor