Korean J Ophthalmol.
2014 Jun;28(3):226-233. 10.3341/kjo.2014.28.3.226.
The Usefulness of Interferon-gamma Release Assay for Diagnosis of Tuberculosis-related Uveitis in Korea
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. sejoon1@snu.ac.kr
- 2Department of Ophthalmology, Armed Forces Capital Hospital, Seongnam, Korea.
- 3Department of Ophthalmology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2133321
- DOI: http://doi.org/10.3341/kjo.2014.28.3.226
Abstract
- PURPOSE
To evaluate the usefulness of the interferon-gamma release assay (IGRA) for diagnosing tuberculosis (TB)-related uveitis (TRU).
METHODS
Records from 181 patients with ocular signs and symptoms suggestive of TRU and intraocular inflammation of unknown etiology were reviewed. All subjects underwent clinical and laboratory testing, including IGRA, to rule out presence of underlying disease. A diagnosis of presumed TRU was made based on an internist's TB diagnosis and a patient's response to anti-TB therapy. Sensitivity, specificity, and positive predictive values of IGRA for TRU diagnosis were calculated. Clinical characteristics were compared between patients with positive and negative IGRA results.
RESULTS
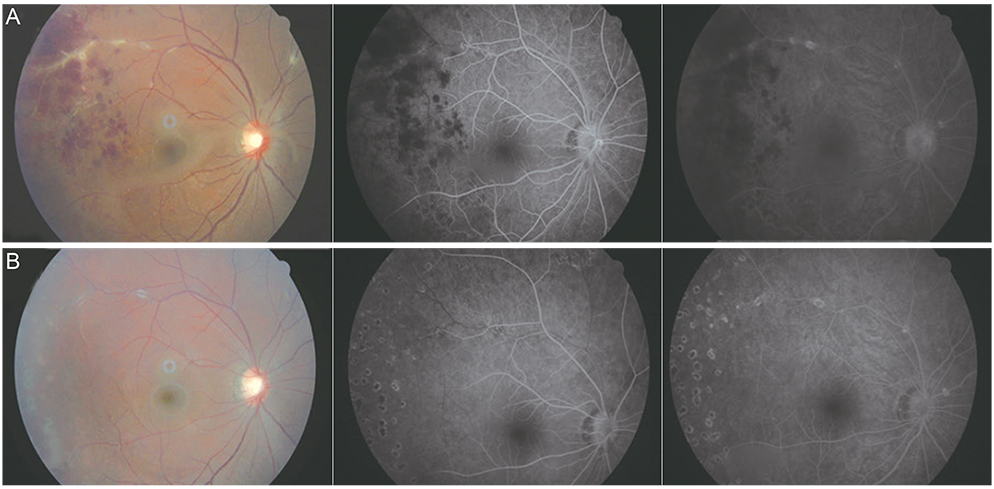
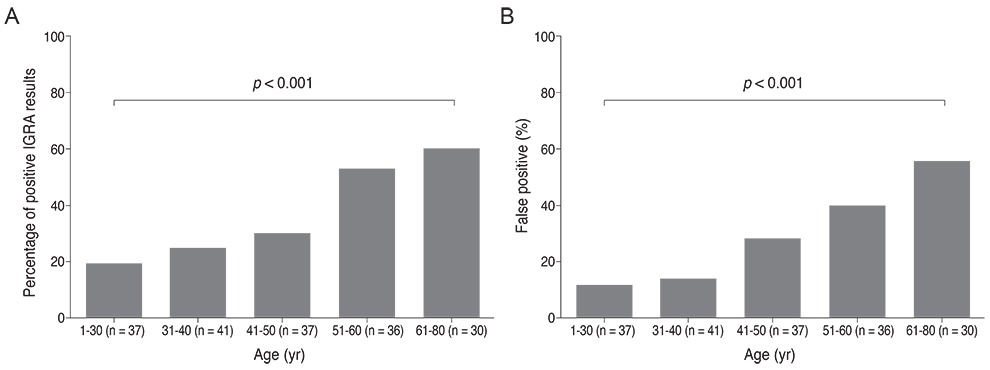
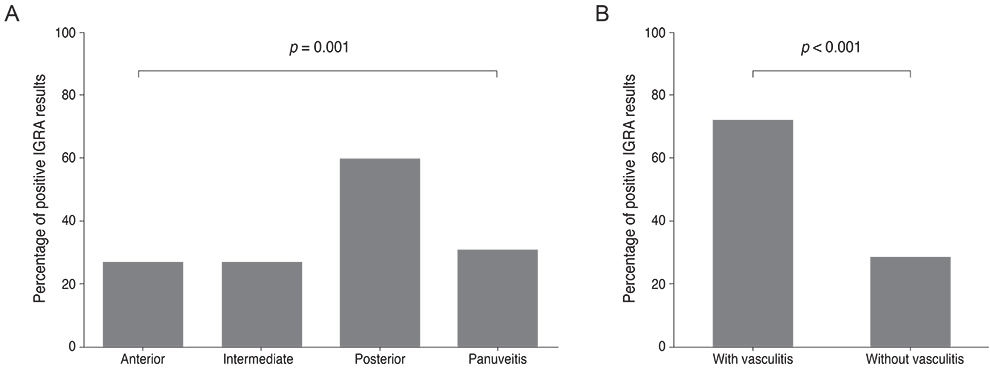
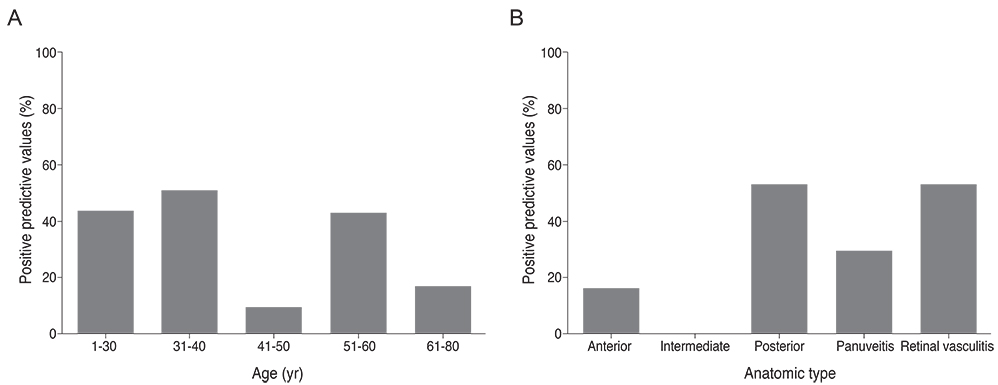
The sensitivity and specificity of IGRA for TRU were 100% and 72.0%, respectively. Mean age, percentage of patients with retinal vasculitis, and the anatomic type of uveitis were significantly different between patients with positive and negative IGRA results (all p < or = 0.001). Positive IGRA rates and false-positive rates were significantly different between age and anatomic type groups (both p = 0.001). The positive predictive value of the IGRA among patients with intraocular inflammation was high (70%) when all of younger age (< or =40 years), posterior uveitis, and retinal vasculitis were present.
CONCLUSIONS
The IGRA is useful for diagnosing TRU in the Korean population, especially when it is used as a screening test. Clinical characteristics, including younger age (< or =40 years), posterior uveitis, and retinal vasculitis in IGRA-positive patients, increase the likelihood of the patient having TRU.
MeSH Terms
-
Adolescent
Adult
Aged
Aged, 80 and over
Child
Female
Humans
Incidence
Interferon-gamma/*analysis
Interferon-gamma Release Tests/*methods
Male
Middle Aged
Predictive Value of Tests
Reproducibility of Results
Republic of Korea/epidemiology
Retrospective Studies
Tuberculosis, Ocular/*diagnosis/epidemiology/microbiology
Uveitis/*diagnosis/epidemiology/microbiology
Young Adult
Interferon-gamma
Figure
Reference
-
1. Centers for Disease Control and Prevention (CDC). Trends in tuberculosis: United States, 2004. MMWR Morb Mortal Wkly Rep. 2005; 54:245–249.2. Dye C, Scheele S, Dolin P, et al. WHO Global Surveillance and Monitoring Project. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999; 282:677–686.3. Centers for Disease Control and Prevention (CDC). Expanded tuberculosis surveillance and tuberculosis morbidity: United States, 1993. MMWR Morb Mortal Wkly Rep. 1994; 43:361–366.4. Tabbara KF. Tuberculosis. Curr Opin Ophthalmol. 2007; 18:493–501.5. Kang YA, Lee HW, Yoon HI, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293:2756–2761.6. Itty S, Bakri SJ, Pulido JS, et al. Initial results of QuantiFERON-TB Gold testing in patients with uveitis. Eye (Lond). 2009; 23:904–909.7. Ang M, Htoon HM, Chee SP. Diagnosis of tuberculous uveitis: clinical application of an interferon-gamma release assay. Ophthalmology. 2009; 116:1391–1396.8. Feng Y, Diao N, Shao L, et al. Interferon-gamma release assay performance in pulmonary and extrapulmonary tuberculosis. PLoS One. 2012; 7:e32652.9. Critselis E, Amanatidou V, Syridou G, et al. The effect of age on whole blood interferon-gamma release assay response among children investigated for latent tuberculosis infection. J Pediatr. 2012; 161:632–638.10. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000; 161(4 Pt 1):1376–1395.11. Mazurek GH, Jereb J, Lobue P, et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep. 2005; 54(RR-15):49–55.12. Mazurek GH, Jereb J, Vernon A, et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010; 59(RR-5):1–25.13. Madariaga MG, Jalali Z, Swindells S. Clinical utility of interferon gamma assay in the diagnosis of tuberculosis. J Am Board Fam Med. 2007; 20:540–547.14. Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002; 359:881–884.15. Gupta A, Bansal R, Gupta V, et al. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. 2010; 149:562–570.16. Gupta A, Gupta V, Arora S, et al. PCR-positive tubercular retinal vasculitis: clinical characteristics and management. Retina. 2001; 21:435–444.17. Gupta V, Gupta A, Rao NA. Intraocular tuberculosis: an update. Surv Ophthalmol. 2007; 52:561–587.18. Hoh HB, Kong VY, Jaais F. Tuberculous retinal vasculitis. Med J Malaysia. 1998; 53:288–289.19. Reny JL, Challe G, Geisert P, et al. Tuberculosis-related retinal vasculitis in an immunocompetent patient. Clin Infect Dis. 1996; 22:873–874.20. Bodaghi B, LeHoang P. Ocular tuberculosis. Curr Opin Ophthalmol. 2000; 11:443–448.21. Ang M, Wong W, Ngan CC, Chee SP. Interferon-gamma release assay as a diagnostic test for tuberculosis-associated uveitis. Eye (Lond). 2012; 26:658–665.22. Lee H, Dockrell HM, Kim DR, et al. The current status of BCG vaccination in young children in South Korea. Tuberc Respir Dis (Seoul). 2012; 72:374–380.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Presumed Latent Tuberculosis-related Intermediate Uveitis Manifesting as Recurrent Vitreous Hemorrhage
- Clinical Usefulness of Combined Anti-tuberculosis Antibody Test and Interferon-gamma Release Assay for the Diagnosis of Tuberculosis
- Interferon-gamma Release Assay Using Pericardial Fluid and Peripheral Blood for the Diagnosis of Tuberculous Pericarditis: A Case Report
- A Case of Lupus Vulgaris Diagnosed with Interferon-gamma Release Assay
- The Usefulness of Whole-blood Interferon-gamma Release Assay for the Diagnosis of Extra-pulmonary Tuberculosis