J Korean Med Sci.
2015 Mar;30(3):301-307. 10.3346/jkms.2015.30.3.301.
Urethroplasty Using Autologous Urethral Tissue-embedded Acellular Porcine Bladder Submucosa Matrix Grafts for the Management of Long-Segment Urethral Stricture in a Rabbit Model
- Affiliations
-
- 1Joint Institute for Regenerative Medicine, Kyungpook National University Hospital, Daegu, Korea.
- 2Department of Urology, School of Medicine, Kyungpook National University, Daegu, Korea. urologistk@knu.ac.kr
- 3Laboratory Animal Center, Yeungnam University, Daegu, Korea.
- 4Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 2133309
- DOI: http://doi.org/10.3346/jkms.2015.30.3.301
Abstract
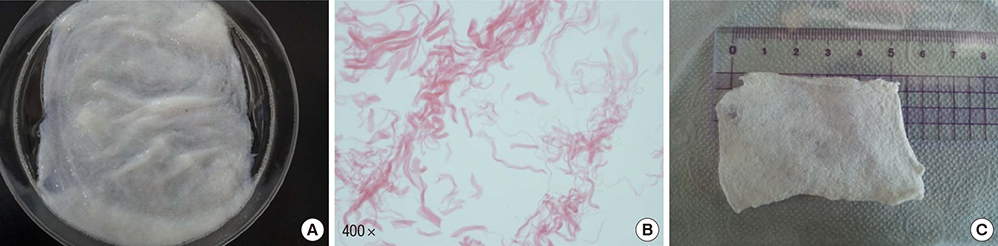
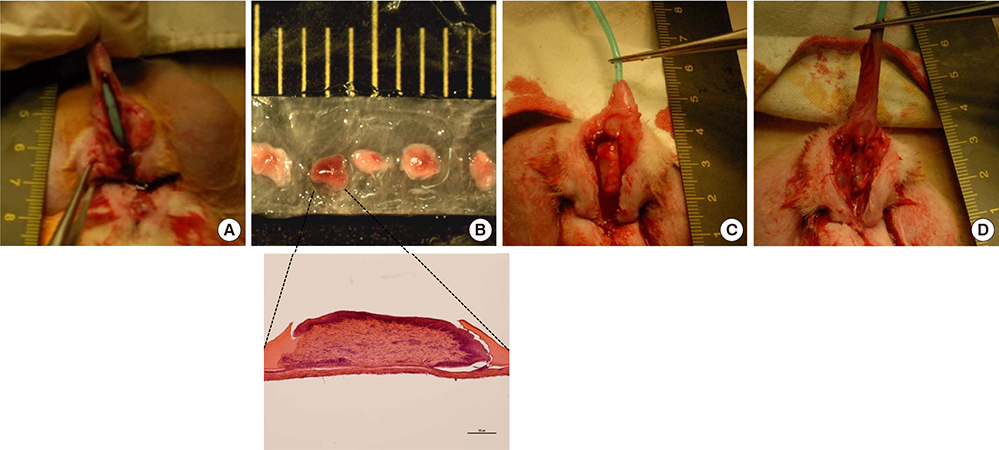
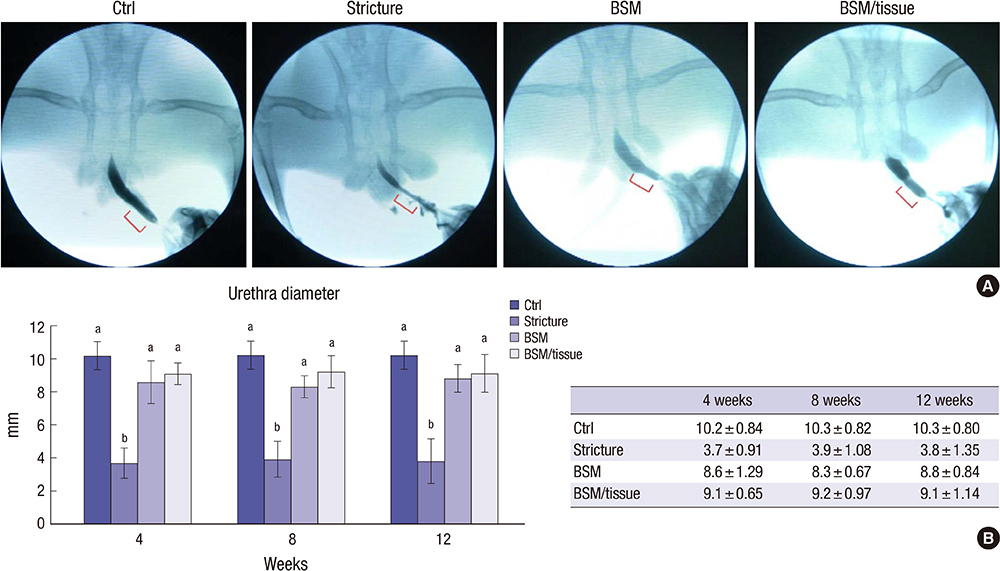
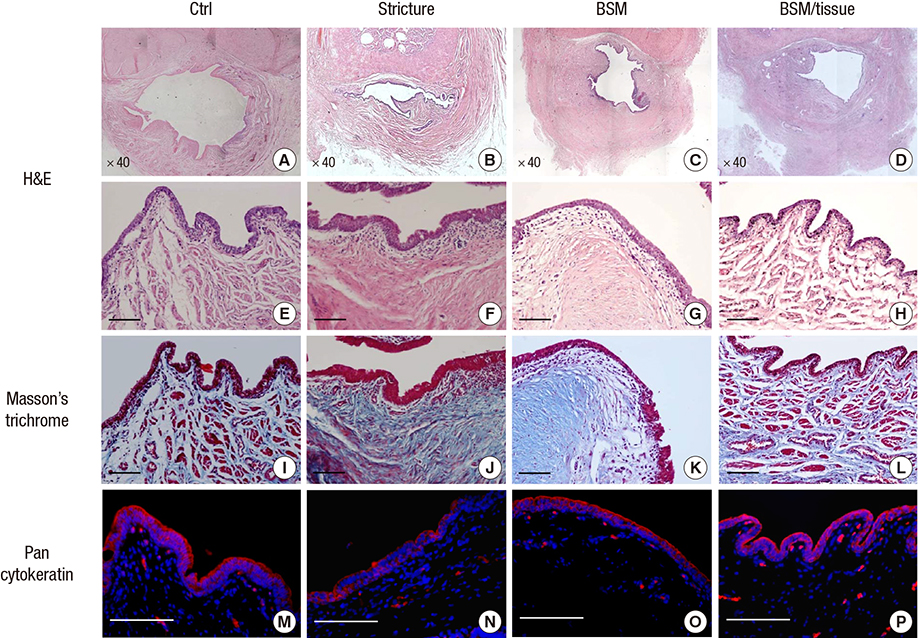
- We conducted this study to evaluate the combined effect of acellular bladder submucosa matrix (BSM) and autologous urethral tissue for the treatment of long segment urethral stricture in a rabbit model. To prepare the BSM, porcine bladder submucosa was processed, decellularized, configured into a sheet-like shape, and sterilized. Twenty rabbits were randomized to normal control, urethral stricture, urethroplasty using BSM only or BSM/autologous urethral tissue (n=5 per group). Retrograde urethrography was performed at 4, 8, and 12 weeks postoperatively, and the grafted specimens were harvested at week 12 to evaluate urethral reconstruction through histopathologic and immunohistochemical analysis. The mean urethral width of the control, stricture, BSM, and BSM/autologous urethral tissue groups at week 12 was 10.3+/-0.80, 3.8+/-1.35, 8.8+/-0.84, and 9.1+/-1.14 mm, respectively. The histopathologic study revealed that the BSM/autologous urethral tissue graft had a normal area of urethral lumen, compact muscular layers, complete epithelialization, and progressive infiltration by vessels in the regenerated urethra. In contrast, the BSM grafts revealed keratinized epithelium, abundant collagenized fibrous connective tissue, and were devoid of bundles of circular smooth muscle. Nontransected ventral onlay-augmented urethroplasty using an acellular BSM scaffold combined with an autologous urethral tissue graft represents a feasible procedure for urethral reconstruction.
Keyword
MeSH Terms
Figure
Reference
-
1. Sievert KD, Selent-Stier C, Wiedemann J, Greiner TO, Amend B, Stenzl A, Feil G, Seibold J. Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol. 2012; 187:1101–1109.2. Fiala R, Vidlar A, Vrtal R, Belej K, Student V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol. 2007; 51:1702–1708.3. Kropp BP, Sawyer BD, Shannon HE, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, Thor KB. Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation, in vitro compliance and contractility. J Urol. 1996; 156:599–607.4. Kropp BP, Ludlow JK, Spicer D, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, Birhle R, Thor KB. Rabbit urethral regeneration using small intestinal submucosa onlay grafts. Urology. 1998; 52:138–142.5. Badylak SF, Vorp DA, Spievack AR, Simmons-Byrd A, Hanke J, Freytes DO, Thapa A, Gilbert TW, Nieponice A. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005; 128:87–97.6. Dahms SE, Piechota HJ, Nunes L, Dahiya R, Lue TF, Tanagho EA. Free ureteral replacement in rats: regeneration of ureteral wall components in the acellular matrix graft. Urology. 1997; 50:818–825.7. Merguerian PA, Reddy PP, Barrieras DJ, Wilson GJ, Woodhouse K, Bagli DJ, McLorie GA, Khoury AE. Acellular bladder matrix allografts in the regeneration of functional bladders: evaluation of large-segment (> 24 cm) substitution in a porcine model. BJU Int. 2000; 85:894–898.8. Probst M, Dahiya R, Carrier S, Tanagho EA. Reproduction of functional smooth muscle tissue and partial bladder replacement. Br J Urol. 1997; 79:505–515.9. Sutherland RS, Baskin LS, Hayward SW, Cunha GR. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J Urol. 1996; 156:571–577.10. Yoo JJ, Meng J, Oberpenning F, Atala A. Bladder augmentation using allogenic bladder submucosa seeded with cells. Urology. 1998; 51:221–225.11. Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int. 2005; 96:1120–1125.12. Chun SY, Lim GJ, Kwon TG, Kwak EK, Kim BW, Atala A, Yoo JJ. Identification and characterization of bioactive factors in bladder submucosa matrix. Biomaterials. 2007; 28:4251–4256.13. Kim BS, Kim HT, Kwon SY, Chun SY, Choi KH, Park M, Kim DH, Song PH, Kwon TG. Nontransected ventral onlay-augmented urethroplasty using autologous saphenous vein graft in a rabbit model of urethral stricture. Urology. 2014; 83:225–231.14. Retik AB, Atala A. Complications of hypospadias repair. Urol Clin North Am. 2002; 29:329–339.15. Freytes DO, Tullius RS, Valentin JE, Stewart-Akers AM, Badylak SF. Hydrated versus lyophilized forms of porcine extracellular matrix derived from the urinary bladder. J Biomed Mater Res A. 2008; 87:862–872.16. Lorenz C, Maier-Reif K, Back W, Pohl HP, Wang KL. Cultured urothelium in sheep bladder augmentation. Pediatr Surg Int. 1996; 11:456–461.17. Morrison WA, Webster HR, Kumta S. Urethral reconstruction using the radial artery forearm free flap: conventional and prefabricated. Plast Reconstr Surg. 1996; 97:413–419.18. Fairbanks JL, Sheldon CA, Khoury AE, Gilbert A, Bove KE. Free bladder mucosal graft biology: unique engraftment characteristics in rabbits. J Urol. 1992; 148:663–666.19. Jung HS, Kim JW, Lee JN, Kim HT, Yoo ES, Kim BS. Early experience with a thermo-expandable stent (memokath) for the management of recurrent urethral stricture. Korean J Urol. 2013; 54:851–857.20. Büyükünal SN, Cerrah A, Dervişoğlu S. Appendix interposition in the treatment of severe posterior urethral injuries. J Urol. 1995; 154:840–843.21. Atala A, Guzman L, Retik AB. A novel inert collagen matrix for hypospadias repair. J Urol. 1999; 162:1148–1151.22. El-Kassaby AW, Retik AB, Yoo JJ, Atala A. Urethral stricture repair with an off-the-shelf collagen matrix. J Urol. 2003; 169:170–173.23. Mantovani F, Trinchieri A, Castelnuovo C, Romanò AL, Pisani E. Reconstructive urethroplasty using porcine acellular matrix. Eur Urol. 2003; 44:600–602.24. Sabbagh W, Masters JR, Duffy PG, Herbage D, Brown RA. In vitro assessment of a collagen sponge for engineering urothelial grafts. Br J Urol. 1998; 82:888–894.25. Pu LL. Discussion. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg. 2009; 124:1447–1449.26. Glotzbach JP, Levi B, Wong VW, Longaker MT, Gurtner GC. The basic science of vascular biology: implications for the practicing surgeon. Plast Reconstr Surg. 2010; 126:1528–1538.27. Vaos G, Gardikis S, Giatromanolaki A, Kambouri K, Triotapsianis G, Ypsilantis P, Sivridis E, Simopoulos C. Long-term angiogenic activity of free grafts and pedicle flap in a rabbit urethroplasty model. World J Urol. 2013; 31:919–924.28. World Health Organization. Essential intervention No. 4: Scar management and control. accessed on 3 February 2015. Available at http://www.who.int/buruli/information/publications/BU-5POD-interventions-4.pdf?ua=1.29. De Filippo RE, Yoo JJ, Atala A. Urethral replacement using cell seeded tubularized collagen matrices. J Urol. 2002; 168:1789–1792.