Anat Cell Biol.
2015 Dec;48(4):225-234. 10.5115/acb.2015.48.4.225.
Role of mast cell in the late phase of contact hypersensitivity induced by trimellitic anhydride
- Affiliations
-
- 1Department of Anatomy, Chonbuk National University Medical School and Institute for Medical Science, Chonbuk National University, Jeonju, Korea. asch@jbnu.ac.kr
- KMID: 2133261
- DOI: http://doi.org/10.5115/acb.2015.48.4.225
Abstract
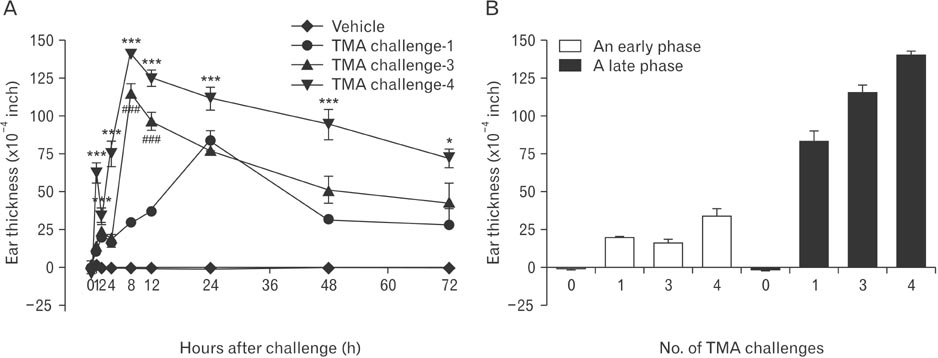
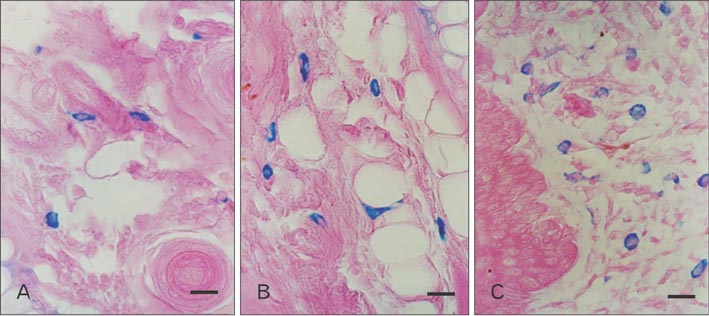
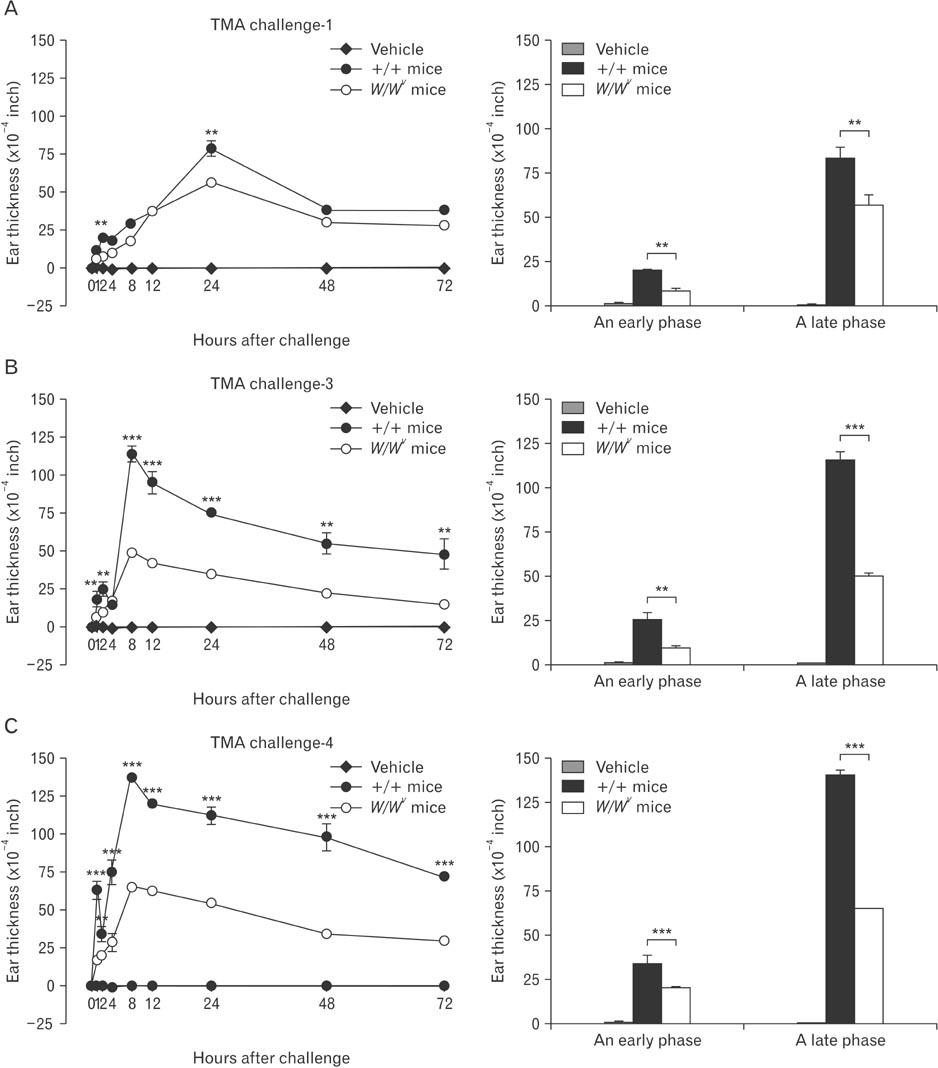
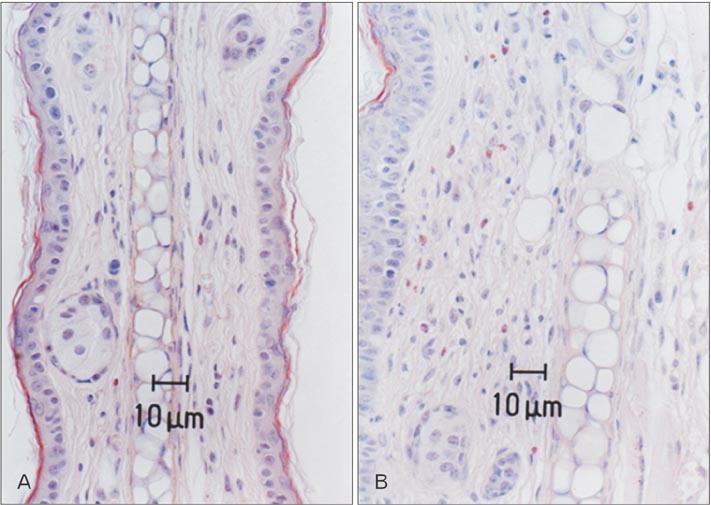
- Mast cells are known as effector cells of IgE-mediated allergic responses, but role of mast cells in contact hypersensitivity (CHS) has been considered controversial. In this study, we investigated role of mast cell in trimellitic anhydride (TMA)-induced CHS. The mice were sensitized to TMA on the back and repeatedly challenged with TMA on the left ear at 1-week intervals. The ear after challenge showed biphasic responses. The repetition of TMA challenge shifted in time course of ear response and enlarged the extent of early and late phase reactions in proportion to the frequency of TMA challenges in C57BL/6 mice. In late phase reaction, peak of ear response by single challenge showed at 24 hours after challenge, but the peak by repeat challenges at 8 hours after the last challenge. Number of mast cells and eosinophils per unit area increased in proportion to frequency of TMA challenges. However, mast cell-deficient WBB6F1/J-Kit(W)/Kit(W-v) mice developed the late phase reaction without the early phase reaction. The repetition of TMA challenge shifted in time course of ear response and enlarged the extent of ear response and the infiltration of eosinophils. The magnitude of these responses observed according to the frequency of the TMA challenge in mast cell-deficient WBB6F1/J-Kit(W)/Kit(W-v) mice was significantly lower than that in C57BL/6 mice. Also TMA elicited mast cell degranulation and histamine release from rat peritoneal mast cells in a concentration-dependent manner. Conclusively, TMA induces the early and late phase reactions in CHS, and mast cells may be required for TMA-induced CHS.
Figure
Reference
-
1. Uter W, Schnuch A, Geier J, Frosch PJ. Epidemiology of contact dermatitis: the information network of departments of dermatology (IVDK) in Germany. Eur J Dermatol. 1998; 8:36–40.2. Saint-Mezard P, Rosieres A, Krasteva M, Berard F, Dubois B, Kaiserlian D, Nicolas JF. Allergic contact dermatitis. Eur J Dermatol. 2004; 14:284–295.3. Ryan CA, Hulette BC, Gerberick GF. Approaches for the development of cell-based in vitro methods for contact sensitization. Toxicol In Vitro. 2001; 15:43–55.4. Inagaki N, Sakurai T, Abe T, Musoh K, Kawasaki H, Tsunematsu M, Nagai H. Characterization of antihistamines using biphasic cutaneous reaction in BALB/c mice. Life Sci. 1998; 63:PL145–PL150.5. Togawa M, Kiniwa M, Nagai H. The roles of IL-4, IL-5 and mast cells in the accumulation of eosinophils during allergic cutaneous late phase reaction in mice. Life Sci. 2001; 69:699–705.6. Zhang XD, Murray DK, Lewis DM, Siegel PD. Dose-response and time course of specific IgE and IgG after single and repeated topical skin exposure to dry trimellitic anhydride powder in a Brown Norway rat model. Allergy. 2002; 57:620–626.7. Dearman RJ, Warbrick EV, Skinner R, Kimber I. Cytokine fingerprinting of chemical allergens: species comparisons and statistical analyses. Food Chem Toxicol. 2002; 40:1881–1892.8. Sailstad DM, Ward MD, Boykin EH, Selgrade MK. A murine model for low molecular weight chemicals: differentiation of respiratory sensitizers (TMA) from contact sensitizers (DNFB). Toxicology. 2003; 194:147–161.9. Schneider C, Döcke WD, Zollner TM, Röse L. Chronic mouse model of TMA-induced contact hypersensitivity. J Invest Dermatol. 2009; 129:899–907.10. Chai OH, Lee HK, Lee YC, Lee MS, Han EH, Kim HT, Song CH. Roles of TNF-alpha and IgE in the late phase of contact hypersensitivity induced by trimellitic anhydride. Exp Mol Med. 2005; 37:408–417.11. Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010; 10:440–452.12. Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007; 19:31–38.13. Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005; 167:835–848.14. McNeil HP, Adachi R, Stevens RL. Mast cell-restricted tryptases: structure and function in inflammation and pathogen defense. J Biol Chem. 2007; 282:20785–20789.15. Kitagaki H, Fujisawa S, Watanabe K, Hayakawa K, Shiohara T. Immediate-type hypersensitivity response followed by a late reaction is induced by repeated epicutaneous application of contact sensitizing agents in mice. J Invest Dermatol. 1995; 105:749–755.16. Galli SJ, Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984; 226:710–713.17. Mekori YA, Galli SJ. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985; 135:879–885.18. Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007; 8:1095–1104.19. Song CH, Galli SJ, Lantz CS, Hu X, Stevens RL, Friend DS. 13 Congo red staining of intraepithelial mucosal mast cells. J Histochem Cytochem. 1999; 47:1645C–11645.20. Chai OH, Shon DH, Han EH, Kim HT, Song CH. Effects of Anemarrhena asphodeloides on IgE-mediated passive cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis and mast cell activation. Exp Toxicol Pathol. 2013; 65:419–426.21. Landsteiner K, Jacobs J. Studies on the sensitization of animals with simple chemical compounds. J Exp Med. 1935; 61:643–656.22. Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006; 118:178–189.23. Sugawara Y, Okamoto Y, Sawahata T, Tanaka K. Skin reactivity in guinea pigs sensitized with 2,4-toluene diisocyanate. Int Arch Allergy Immunol. 1993; 100:190–196.24. Dvorak HF, Mihm MC Jr, Dvorak AM. Morphology of delayed-type hypersensitivity reactions in man. J Invest Dermatol. 1976; 67:391–401.25. Arts JH, Dröge SC, Bloksma N, Kuper CF. Local lymph node activation in rats after dermal application of the sensitizers 2,4-dinitrochlorobenzene and trimellitic anhydride. Food Chem Toxicol. 1996; 34:55–62.26. Ban M, Hettich D. Relationship between IgE positive cell numbers and serum total IgE levels in mice treated with trimellitic anhydride and dinitrochlorobenzene. Toxicol Lett. 2001; 118:129–137.27. Vento KL, Dearman RJ, Kimber I, Basketter DA, Coleman JW. Selectivity of IgE responses, mast cell sensitization, and cytokine expression in the immune response of Brown Norway rats to chemical allergens. Cell Immunol. 1996; 172:246–253.28. Sawada K, Nagai H, Basaki Y, Yamaya H, Ikizawa K, Watanabe M, Kojima M, Matsuura N, Kiniwa M. The expression of murine cutaneous late phase reaction requires both IgE antibodies and CD4 T cells. Clin Exp Allergy. 1997; 27:225–231.29. Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008; 213:251–260.30. Seder RA, Paul WE, Ben-Sasson SZ, LeGros GS, Kagey-Sobotka A, Finkelman FD, Pierce JH, Plaut M. Production of interleukin-4 and other cytokines following stimulation of mast cell lines and in vivo mast cells/basophils. Int Arch Allergy Appl Immunol. 1991; 94:137–140.31. He S, Walls AF. The induction of a prolonged increase in microvascular permeability by human mast cell chymase. Eur J Pharmacol. 1998; 352:91–98.32. He S, Walls AF. Human mast cell chymase induces the accumulation of neutrophils, eosinophils and other inflammatory cells in vivo. Br J Pharmacol. 1998; 125:1491–1500.33. Tomimori Y, Tsuruoka N, Fukami H, Saito K, Horikawa C, Saito M, Muto T, Sugiura N, Yamashiro K, Sumida M, Kakutani S, Fukuda Y. Role of mast cell chymase in allergen-induced biphasic skin reaction. Biochem Pharmacol. 2002; 64:1187.34. Natsuaki M, Yano N, Yamaya K, Kitano Y. Immediate contact hypersensitivity induced by repeated hapten challenge in mice. Contact Dermatitis. 2000; 43:267–272.35. Dearman RJ, Basketter DA, Kimber I. Variable effects of chemical allergens on serum IgE concentration in mice: preliminary evaluation of a novel approach to the identification of respiratory sensitizers. J Appl Toxicol. 1992; 12:317–323.36. Dearman RJ, Basketter DA, Kimber I. Selective induction of type 2 cytokines following topical exposure of mice to platinum salts. Food Chem Toxicol. 1998; 36:199–207.37. Kitagaki H, Ono N, Hayakawa K, Kitazawa T, Watanabe K, Shiohara T. Repeated elicitation of contact hypersensitivity induces a shift in cutaneous cytokine milieu from a T helper cell type 1 to a T helper cell type 2 profile. J Immunol. 1997; 159:2484–2491.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of IL-10 in the Trimellitic Anhydride-induced Contact Dermatitis
- Roles of TNF-alpha and IgE in the late phase of contact hypersensitivity induced by trimellitic anhydride
- A Murine Model of Toluene Diisocyanate-induced Contact Hypersensitivity
- Inhibitory Effect of Disosium Cromoglycate and Ketotifen on Human Seminal Plasma-Induced Mast Cell Activation
- Mast Cells and Allergic Rhinitis