Yonsei Med J.
2014 Jul;55(4):1014-1027. 10.3349/ymj.2014.55.4.1014.
Identification of Pancreatic Cancer-Associated Tumor Antigen from HSP-Enriched Tumor Lysate-Pulsed Human Dendritic Cells
- Affiliations
-
- 1Innovative Cell and Gene Therapy Center, International St. Mary's Hospital, Incheon, Korea.
- 2Center for Bioanalysis, Division of Metrology for Quality of Life, Korea Research Institute of Standards and Science, Daejeon, Korea.
- 3Department of Chemistry, Yonsei University, Seoul, Korea. mhmoon@yonsei.ac.kr
- 4Department of Internal Medicine, Hallym University College of Medicine, Chuncheon, Korea. hyung@hallym.or.kr
- KMID: 2130828
- DOI: http://doi.org/10.3349/ymj.2014.55.4.1014
Abstract
- PURPOSE
Vaccine strategies utilizing dendritic cells (DCs) to elicit anti-tumor immunity are the subject of intense research. Although we have shown that DCs pulsed with heat-treated tumor lysate (HTL) induced more potent anti-tumor immunity than DCs pulsed with conventional tumor lysate (TL), the underlying molecular mechanism is unclear. In order to explore the molecular basis of this approach and to identify potential antigenic peptides from pancreatic cancer, we analyzed and compared the major histocompatibility complex (MHC) ligands derived from TL- and HTL-pulsed dendritic cells by mass spectrophotometry.
MATERIALS AND METHODS
Human monocyte-derived dendritic cells were pulsed with TL or HTL prior to maturation induction. To delineate differences of MHC-bound peptide repertoire eluted from DCs pulsed with TL or HTL, nanoflow liquid chromatography-electrospray ionization-tandem mass spectrometry (nLC-ESI-MS-MS) was employed.
RESULTS
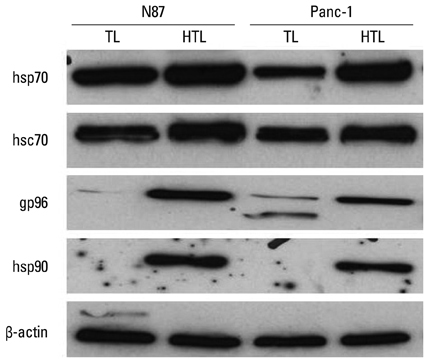
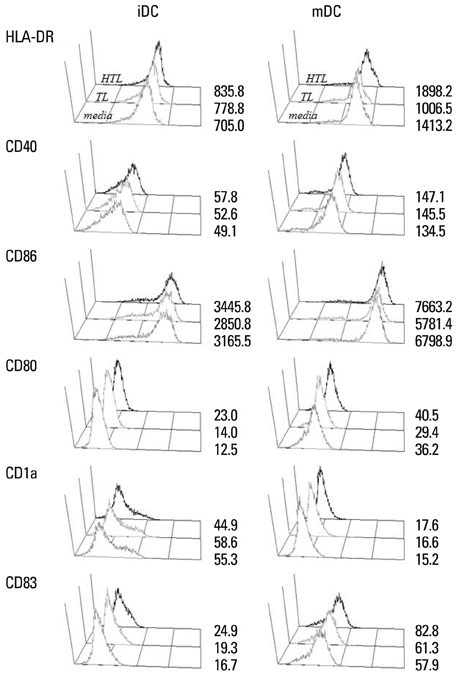
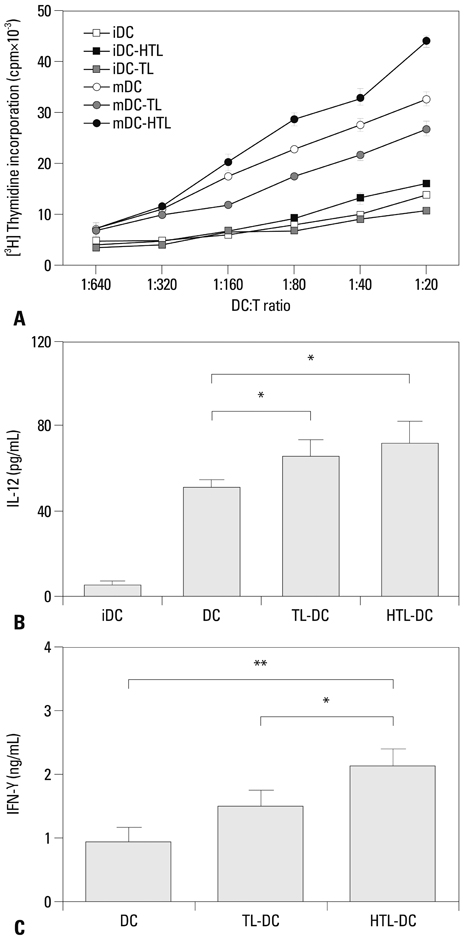
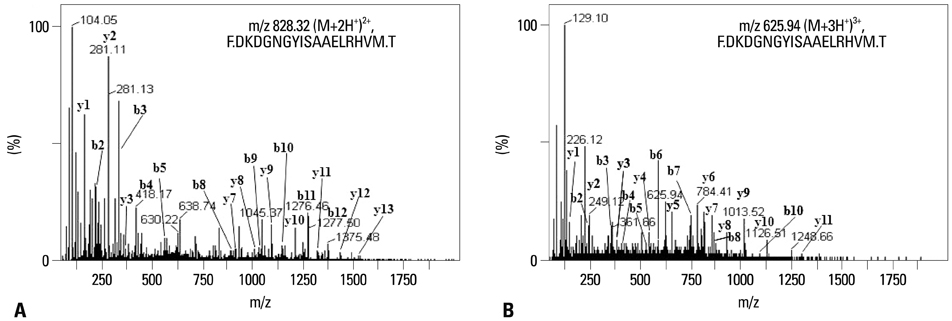
HTL, but not TL, significantly induced DC function, assessed by phenotypic maturation, allostimulation capacity and IFN-gamma secretion by stimulated allogeneic T cells. DCs pulsed with TL or HTL displayed pancreas or pancreatic cancer-related peptides in context of MHC class I and II molecules. Some of the identified peptides had not been previously reported as expressed in pancreatic cancer or cancer of other tissue types.
CONCLUSION
Our partial lists of MHC-associated peptides revealed the differences between peptide profiles eluted from HTL-and TL-loaded DCs, implying that induced heat shock proteins in HTL chaperone tumor-derived peptides enhanced their delivery to DCs and promoted cross-presentation by DC. These findings may aid in identifying novel tumor antigens or biomarkers and in designing future vaccination strategies.
MeSH Terms
Figure
Reference
-
1. Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G, et al. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods. 2002; 268:131–140.
Article2. Kim S, Kim HO, Baek EJ, Choi Y, Kim HS, Lee MG. Monocyte enrichment from leukapheresis products by using the Elutra cell separator. Transfusion. 2007; 47:2290–2296.
Article3. Schreurs MW, Eggert AA, de Boer AJ, Vissers JL, van Hall T, Offringa R, et al. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 2000; 60:6995–7001.4. Hofmann S, Glückmann M, Kausche S, Schmidt A, Corvey C, Lichtenfels R, et al. Rapid and sensitive identification of major histocompatibility complex class I-associated tumor peptides by Nano-LC MALDI MS/MS. Mol Cell Proteomics. 2005; 4:1888–1897.
Article5. Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998; 393:474–478.
Article6. Hu HM, Winter H, Urba WJ, Fox BA. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J Immunol. 2000; 165:4246–4253.
Article7. Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001; 22:269–276.
Article8. Schuurhuis DH, Laban S, Toes RE, Ricciardi-Castagnoli P, Kleijmeer MJ, van der Voort EI, et al. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell-independent or -dependent stimuli. J Exp Med. 2000; 192:145–150.
Article9. Melief CJ, Van Der Burg SH, Toes RE, Ossendorp F, Offringa R. Effective therapeutic anticancer vaccines based on precision guiding of cytolytic T lymphocytes. Immunol Rev. 2002; 188:177–182.
Article10. Dolan BP, Gibbs KD Jr, Ostrand-Rosenberg S. Tumor-specific CD4+ T cells are activated by "cross-dressed" dendritic cells presenting peptide-MHC class II complexes acquired from cell-based cancer vaccines. J Immunol. 2006; 176:1447–1455.
Article11. Parmiani G, Castelli C, Dalerba P, Mortarini R, Rivoltini L, Marincola FM, et al. Cancer immunotherapy with peptide-based vaccines: what have we achieved? Where are we going? J Natl Cancer Inst. 2002; 94:805–818.
Article12. Admon A, Barnea E, Ziv T. Tumor antigens and proteomics from the point of view of the major histocompatibility complex peptides. Mol Cell Proteomics. 2003; 2:388–398.
Article13. Fields RC, Shimizu K, Mulé JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci U S A. 1998; 95:9482–9487.
Article14. Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998; 4:328–332.
Article15. Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, et al. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001; 61:6445–6450.16. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004; 64:4973–4979.
Article17. Geiger JD, Hutchinson RJ, Hohenkirk LF, McKenna EA, Yanik GA, Levine JE, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001; 61:8513–8519.18. Höltl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, et al. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002; 8:3369–3376.19. Hatfield P, Merrick AE, West E, O'Donnell D, Selby P, Vile R, et al. Optimization of dendritic cell loading with tumor cell lysates for cancer immunotherapy. J Immunother. 2008; 31:620–632.
Article20. Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000; 191:423–434.21. Kotera Y, Shimizu K, Mulé JJ. Comparative analysis of necrotic and apoptotic tumor cells as a source of antigen(s) in dendritic cell-based immunization. Cancer Res. 2001; 61:8105–8109.22. Galea-Lauri J, Wells JW, Darling D, Harrison P, Farzaneh F. Strategies for antigen choice and priming of dendritic cells influence the polarization and efficacy of antitumor T-cell responses in dendritic cell-based cancer vaccination. Cancer Immunol Immunother. 2004; 53:963–977.
Article23. Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000; 191:411–416.
Article24. Kim HS, Choo YS, Koo T, Bang S, Oh TY, Wen J, et al. Enhancement of antitumor immunity of dendritic cells pulsed with heat-treated tumor lysate in murine pancreatic cancer. Immunol Lett. 2006; 103:142–148.
Article25. Qiu J, Li GW, Sui YF, Song HP, Si SY, Ge W. Heat-shocked tumor cell lysate-pulsed dendritic cells induce effective anti-tumor immune response in vivo. World J Gastroenterol. 2006; 12:473–478.
Article26. Bachleitner-Hofmann T, Stift A, Friedl J, Pfragner R, Radelbauer K, Dubsky P, et al. Stimulation of autologous antitumor T-cell responses against medullary thyroid carcinoma using tumor lysate-pulsed dendritic cells. J Clin Endocrinol Metab. 2002; 87:1098–1104.
Article27. Arnold-Schild D, Hanau D, Spehner D, Schmid C, Rammensee HG, de la Salle H, et al. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999; 162:3757–3760.28. Babatz J, Röllig C, Oelschlägel U, Zhao S, Ehninger G, Schmitz M, et al. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: a phase I study. J Hematother Stem Cell Res. 2003; 12:515–523.
Article29. Santin AD, Bellone S, Ravaggi A, Pecorelli S, Cannon MJ, Parham GP. Induction of ovarian tumor-specific CD8+ cytotoxic T lymphocytes by acid-eluted peptide-pulsed autologous dendritic cells. Obstet Gynecol. 2000; 96:422–430.
Article30. Kang D, Moon MH. Development of non-gel-based two-dimensional separation of intact proteins by an on-line hyphenation of capillary isoelectric focusing and hollow fiber flow field-flow fractionation. Anal Chem. 2006; 78:5789–5798.
Article31. Kang D, Ji ES, Moon MH, Yoo JS. Lectin-based enrichment method for glycoproteomics using hollow fiber flow field-flow fractionation: application to Streptococcus pyogenes. J Proteome Res. 2010; 9:2855–2862.
Article32. Srivastava PK. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr Oncol Rep. 2005; 7:104–108.
Article33. Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000; 30:705–708.
Article34. Reynolds JL, Mahajan SD, Aalinkeel R, Nair B, Sykes DE, Schwartz SA. Proteomic analyses of the effects of drugs of abuse on monocyte-derived mature dendritic cells. Immunol Invest. 2009; 38:526–550.
Article35. Reynolds JL, Mahajan SD, Sykes DE, Schwartz SA, Nair MP. Proteomic analyses of methamphetamine (METH)-induced differential protein expression by immature dendritic cells (IDC). Biochim Biophys Acta. 2007; 1774:433–442.
Article36. Horlock C, Shakib F, Mahdavi J, Jones NS, Sewell HF, Ghaemmaghami AM. Analysis of proteomic profiles and functional properties of human peripheral blood myeloid dendritic cells, monocyte-derived dendritic cells and the dendritic cell-like KG-1 cells reveals distinct characteristics. Genome Biol. 2007; 8:R30.
Article37. Watarai H, Hinohara A, Nagafune J, Nakayama T, Taniguchi M, Yamaguchi Y. Plasma membrane-focused proteomics: dramatic changes in surface expression during the maturation of human dendritic cells. Proteomics. 2005; 5:4001–4011.
Article38. McIlroy D, Tanguy-Royer S, Le Meur N, Guisle I, Royer PJ, Léger J, et al. Profiling dendritic cell maturation with dedicated microarrays. J Leukoc Biol. 2005; 78:794–803.
Article39. Pereira SR, Faça VM, Gomes GG, Chammas R, Fontes AM, Covas DT, et al. Changes in the proteomic profile during differentiation and maturation of human monocyte-derived dendritic cells stimulated with granulocyte macrophage colony stimulating factor/interleukin-4 and lipopolysaccharide. Proteomics. 2005; 5:1186–1198.
Article40. Rivollier A, Perrin-Cocon L, Luche S, Diemer H, Strub JM, Hanau D, et al. High expression of antioxidant proteins in dendritic cells: possible implications in atherosclerosis. Mol Cell Proteomics. 2006; 5:726–736.41. Ferret-Bernard S, Curwen RS, Mountford AP. Proteomic profiling reveals that Th2-inducing dendritic cells stimulated with helminth antigens have a 'limited maturation' phenotype. Proteomics. 2008; 8:980–993.
Article42. Ferreira GB, van Etten E, Lage K, Hansen DA, Moreau Y, Workman CT, et al. Proteome analysis demonstrates profound alterations in human dendritic cell nature by TX527, an analogue of vitamin D. Proteomics. 2009; 9:3752–3764.
Article43. Gundacker NC, Haudek VJ, Wimmer H, Slany A, Griss J, Bochkov V, et al. Cytoplasmic proteome and secretome profiles of differently stimulated human dendritic cells. J Proteome Res. 2009; 8:2799–2811.
Article44. Shiwa M, Nishimura Y, Wakatabe R, Fukawa A, Arikuni H, Ota H, et al. Rapid discovery and identification of a tissue-specific tumor biomarker from 39 human cancer cell lines using the SELDI ProteinChip platform. Biochem Biophys Res Commun. 2003; 309:18–25.
Article45. Park T, Chen ZP, Leavitt J. Activation of the leukocyte plastin gene occurs in most human cancer cells. Cancer Res. 1994; 54:1775–1781.46. Shimada H, Shiratori T, Yasuraoka M, Kagaya A, Kuboshima M, Nomura F, et al. Identification of Makorin 1 as a novel SEREX antigen of esophageal squamous cell carcinoma. BMC Cancer. 2009; 9:232.
Article47. Li YN, Zhang L, Li XL, Cui DJ, Zheng HD, Yang SY, et al. Glycoprotein nonmetastatic B as a prognostic indicator in small cell lung cancer. APMIS. 2014; 122:140–146.
Article48. Kabbage M, Chahed K, Hamrita B, Guillier CL, Trimeche M, Remadi S, et al. Protein alterations in infiltrating ductal carcinomas of the breast as detected by nonequilibrium pH gradient electrophoresis and mass spectrometry. J Biomed Biotechnol. 2008; 2008:564127.
Article49. Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, et al. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002; 123:1478–1484.
Article50. Shen J, Person MD, Zhu J, Abbruzzese JL, Li D. Protein expression profiles in pancreatic adenocarcinoma compared with normal pancreatic tissue and tissue affected by pancreatitis as detected by two-dimensional gel electrophoresis and mass spectrometry. Cancer Res. 2004; 64:9018–9026.
Article51. Melle C, Ernst G, Escher N, Hartmann D, Schimmel B, Bleul A, et al. Protein profiling of microdissected pancreas carcinoma and identification of HSP27 as a potential serum marker. Clin Chem. 2007; 53:629–635.
Article52. Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002; 32:826–836.
Article53. Wang B, Sun J, Kitamoto S, Yang M, Grubb A, Chapman HA, et al. Cathepsin S controls angiogenesis and tumor growth via matrix-derived angiogenic factors. J Biol Chem. 2006; 281:6020–6029.
Article54. Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, et al. Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002; 160:45–50.
Article55. Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996; 183:283–287.
Article56. Hoffmann TK, Meidenbauer N, Dworacki G, Kanaya H, Whiteside TL. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 2000; 60:3542–3549.57. Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, et al. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci U S A. 2000; 97:2715–2718.
Article58. Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997; 186:1177–1182.
Article59. Cranmer LD, Trevor KT, Hersh EM. Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother. 2004; 53:275–306.
Article60. Kammerer R, Stober D, Riedl P, Oehninger C, Schirmbeck R, Reimann J. Noncovalent association with stress protein facilitates cross-priming of CD8+ T cells to tumor cell antigens by dendritic cells. J Immunol. 2002; 168:108–117.
Article61. Delneste Y. Scavenger receptors and heat-shock protein-mediated antigen cross-presentation. Biochem Soc Trans. 2004; 32(Pt 4):633–635.
Article62. Becker T, Hartl FU, Wieland F. CD40, an extracellular receptor for binding and uptake of Hsp70-peptide complexes. J Cell Biol. 2002; 158:1277–1285.
Article63. Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001; 14:303–313.
Article64. Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004; 172:6087–6092.
Article65. Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N, et al. Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine. Cancer Res. 2005; 65:4947–4954.
Article66. Kurotaki T, Tamura Y, Ueda G, Oura J, Kutomi G, Hirohashi Y, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007; 179:1803–1813.
Article67. Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000; 12:1539–1546.
Article68. Somersan S, Larsson M, Fonteneau JF, Basu S, Srivastava P, Bhardwaj N. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001; 167:4844–4852.
Article69. Takakura Y, Takemoto S, Nishikawa M. Hsp-based tumor vaccines: state-of-the-art and future directions. Curr Opin Mol Ther. 2007; 9:385–391.70. Feng H, Zeng Y, Whitesell L, Katsanis E. Stressed apoptotic tumor cells express heat shock proteins and elicit tumor-specific immunity. Blood. 2001; 97:3505–3512.
Article71. Hashemi SM, Hassan ZM, Soudi S, Ghazanfari T, Kheirandish M, Shahabi S. Evaluation of anti-tumor effects of tumor cell lysate enriched by HSP-70 against fibrosarcoma tumor in BALB/c mice. Int Immunopharmacol. 2007; 7:920–927.
Article72. Urban RG, Chicz RM, Lane WS, Strominger JL, Rehm A, Kenter MJ, et al. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc Natl Acad Sci U S A. 1994; 91:1534–1538.
Article73. Stryhn A, Pedersen LO, Holm A, Buus S. Longer peptide can be accommodated in the MHC class I binding site by a protrusion mechanism. Eur J Immunol. 2000; 30:3089–3099.
Article74. Weinschenk T, Gouttefangeas C, Schirle M, Obermayr F, Walter S, Schoor O, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002; 62:5818–5827.75. Hickman HD, Luis AD, Buchli R, Few SR, Sathiamurthy M, VanGundy RS, et al. Toward a definition of self: proteomic evaluation of the class I peptide repertoire. J Immunol. 2004; 172:2944–2952.
Article76. Alldinger I, Dittert D, Peiper M, Fusco A, Chiappetta G, Staub E, et al. Gene expression analysis of pancreatic cell lines reveals genes overexpressed in pancreatic cancer. Pancreatology. 2005; 5:370–379.
Article77. Grønborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, et al. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006; 5:157–171.
Article78. Uozumi N, Gao C, Yoshioka T, Nakano M, Moriwaki K, Nakagawa T, et al. Identification of a novel type of CA19-9 carrier in human bile and sera of cancer patients: an implication of the involvement in nonsecretory exocytosis. J Proteome Res. 2010; 9:6345–6353.
Article79. Storr SJ, Zaitoun AM, Arora A, Durrant LG, Lobo DN, Madhusudan S, et al. Calpain system protein expression in carcinomas of the pancreas, bile duct and ampulla. BMC Cancer. 2012; 12:511.
Article80. Ohnami S, Matsumoto N, Nakano M, Aoki K, Nagasaki K, Sugimura T, et al. Identification of genes showing differential expression in antisense K-ras-transduced pancreatic cancer cells with suppressed tumorigenicity. Cancer Res. 1999; 59:5565–5571.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor effects of Toxoplasma gondii and antigen-pulsed dendritic cells in mice bearing breast cancer
- Mechanism of Differential Ag-specific Immune Induction by Different Tumor Cell Lysate Pulsed DC
- Cytotoxicities of Tumor-specific T Lymphocytes Primed by Glioma Apoptotic Body-or Glioma Cell Lysate-pulsed Dendritic Cells
- Effect of Dendritic Cell Based Cancer Vaccine Using Allogeneic Tumor Cell Lysate in Melanoma Pulmonary Metastasis Model
- Immunocell Therapy for Lung Cancer: Dendritic Cell Based Adjuvant Therapy in Mouse Lung Cancer Model