Clin Exp Otorhinolaryngol.
2015 Dec;8(4):329-334. 10.3342/ceo.2015.8.4.329.
Cochlear Implantation for Profound Hearing Loss After Multimodal Treatment for Neuroblastoma in Children
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. moon.iljoon@gmail.com
- KMID: 2128905
- DOI: http://doi.org/10.3342/ceo.2015.8.4.329
Abstract
OBJECTIVES
Neuroblastoma (NBL) predominantly affects children under 5 years of age. Through multimodal therapy, including chemotherapy, radiotherapy, surgery, and peripheral blood stem cell transplantation, the survival rate in patients with NBL have improved while treatment-related complications have also increased. Treatment-related ototoxicity, mainly from cisplatin, can result in profound hearing loss requiring cochlear implantation (CI). We analyzed the effectiveness and hearing preservation of CI recipients who had treated with multimodal therapy due to NBL.
METHODS
Patients who received multimodal therapy for NBL and subsequent CIs were enrolled. A detailed review of the perioperative hearing test, speech evaluation, and posttreatment complications was conducted. Speech performance was analyzed using the category of auditory performance (CAP) score and the postoperative hearing preservation of low frequencies was also compared. Patients who were candidates for electro-acoustic stimulation (EAS) used an EAS electrode for low frequency hearing preservation.
RESULTS
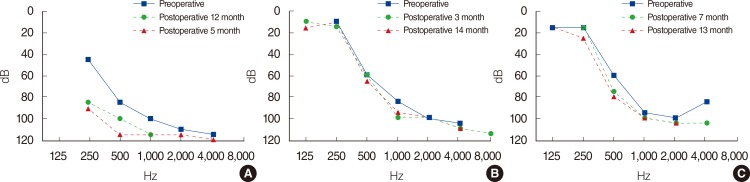
Three patients were identified and all patients showed improvement of speech performance after CI. The average of CAP score improved from 4.3 preoperatively to 5.8 at 1 year postoperatively. Two patients who were fitted with the Flex electrode showed complete hearing preservation and the preserved hearing was maintained over 1 year. The one remaining patient was given the standard CI-512 electrode and showed partial hearing preservation.
CONCLUSION
Patients with profound hearing loss resulting from NBL multimodal therapy can be good candidates for CI, especially for EAS. A soft surgical technique as well as a specifically designed electrode should be applied to this specific population during the CI operation in order to preserve residual hearing and achieve better outcomes.
MeSH Terms
Figure
Reference
-
1. Heck JE, Ritz B, Hung RJ, Hashibe M, Boffetta P. The epidemiology of neuroblastoma: a review. Paediatr Perinat Epidemiol. 2009; 3. 23(2):125–143. PMID: 19159399.
Article2. Cohen LE, Gordon JH, Popovsky EY, Gunawardene S, Duffey-Lind E, Lehmann LE, et al. Late effects in children treated with intensive multimodal therapy for high-risk neuroblastoma: high incidence of endocrine and growth problems. Bone Marrow Transplant. 2014; 4. 49(4):502–508. PMID: 24442245.
Article3. Landier W, Knight K, Wong FL, Lee J, Thomas O, Kim H, et al. Ototoxicity in children with high-risk neuroblastoma: prevalence, risk factors, and concordance of grading scales--a report from the Children's Oncology Group. J Clin Oncol. 2014; 2. 32(6):527–534. PMID: 24419114.
Article4. Brown RF, Hullar TE, Cadieux JH, Chole RA. Residual hearing preservation after pediatric cochlear implantation. Otol Neurotol. 2010; 10. 31(8):1221–1226. PMID: 20818293.
Article5. Raveh E, Attias J, Nageris B, Kornreich L, Ulanovski D. Pattern of hearing loss following cochlear implantation. Eur Arch Otorhinolaryngol. 2015; 9. 272(9):2261–2266. PMID: 25012703.
Article6. Moreno L, Vaidya SJ, Pinkerton CR, Lewis IJ, Imeson J, Machin D, et al. Long-term follow-up of children with high-risk neuroblastoma: the ENSG5 trial experience. Pediatr Blood Cancer. 2013; 7. 60(7):1135–1140. PMID: 23281263.
Article7. Gurney JG, Ross JA, Wall DA, Bleyer WA, Severson RK, Robison LL. Infant cancer in the U.S.: histology-specific incidence and trends, 1973 to 1992. J Pediatr Hematol Oncol. 1997; Sep-Oct. 19(5):428–432. PMID: 9329464.8. Spix C, Pastore G, Sankila R, Stiller CA, Steliarova-Foucher E. Neuroblastoma incidence and survival in European children (1978-1997): report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006; 9. 42(13):2081–2091. PMID: 16919772.
Article9. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993; 8. 11(8):1466–1477. PMID: 8336186.
Article10. Brock PR, Knight KR, Freyer DR, Campbell KC, Steyger PS, Blakley BW, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012; 7. 30(19):2408–2417. PMID: 22547603.
Article11. Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML, et al. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics. 2007; 11. 120(5):e1229–e1236. PMID: 17974716.
Article12. Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr Blood Cancer. 2012; 7. 59(1):144–148. PMID: 22431292.
Article13. Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics. 2010; 4. 125(4):e938–e950. PMID: 20194279.
Article14. Roland JT Jr, Cosetti M, Liebman T, Waltzman S, Allen JC. Cochlear implantation following treatment for medulloblastoma. Laryngoscope. 2010; 1. 120(1):139–143. PMID: 19693928.
Article15. Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005; 12. 23(34):8588–8596. PMID: 16314621.
Article16. Al-Khatib T, Cohen N, Carret AS, Daniel S. Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol. 2010; 8. 74(8):913–919. PMID: 20846503.
Article17. Harris MS, Gilbert JL, Lormore KA, Musunuru SA, Fritsch MH. Cisplatin ototoxicity affecting cochlear implant benefit. Otol Neurotol. 2011; 8. 32(6):969–972. PMID: 21730884.
Article