J Adv Prosthodont.
2014 Jun;6(3):207-214. 10.4047/jap.2014.6.3.207.
Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles
- Affiliations
-
- 1Department of Dentistry, Dongsan Medical Center, School of Medicine, Keimyung University, Daegu, Republic of Korea. nkyp@dsmc.or.kr
- KMID: 2118232
- DOI: http://doi.org/10.4047/jap.2014.6.3.207
Abstract
- PURPOSE
This study characterized the synthesis of a modified PMMA (Polymethyl methacrylate) denture acrylic loading platinum nanoparticles (PtN) and assessed its bacterial inhibitory efficacy to produce novel antimicrobial denture base material.
MATERIALS AND METHODS
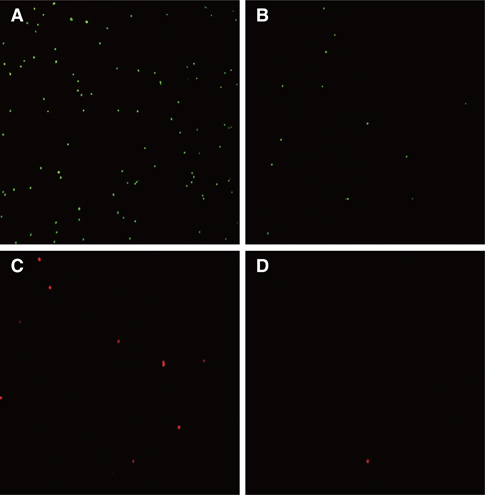
Polymerized PMMA denture acrylic disc (20 mm x 2 mm) specimens containing 0 (control), 10, 50, 100 and 200 mg/L of PtN were fabricated respectively. The obtained platinum-PMMA nanocomposite (PtNC) was characterized by TEM (transmission electron microscopy), SEM/EDX (scanning electron microscope/energy dispersive X-ray spectroscopy), thermogravimetric and atomic absorption spectrophotometer analysis. In antimicrobial assay, specimens were placed on the cell culture plate, and 100 microL of microbial suspensions of S. mutans (Streptococcus mutans) and S. sobrinus (Streptococcus sobrinus) were inoculated then incubated at 37degrees C for 24 hours. The bacterial attachment was tested by FACS (fluorescence-activated cell sorting) analysis after staining with fluorescent probe.
RESULTS
PtN were successfully loaded and uniformly immobilized into PMMA denture acrylic with a proper thermal stability and similar surface morphology as compared to control. PtNC expressed significant bacterial anti-adherent effect rather than bactericidal effect above 50 mg/L PtN loaded when compared to pristine PMMA (P=.01) with no or extremely small amounts of Pt ion eluted.
CONCLUSION
This is the first report on the synthesis and its antibacterial activity of Pt-PMMA nanocomposite. PMMA denture acrylic loading PtN could be a possible intrinsic antimicrobial denture material with proper mechanical characteristics, meeting those specified for denture bases. For clinical application, future studies including biocompatibility, color stability and warranting the long-term effect were still required.
MeSH Terms
Figure
Reference
-
1. Yildirim MS, Hasanreisoglu U, Hasirci N, Sultan N. Adherence of Candida albicans to glow-discharge modified acrylic denture base polymers. J Oral Rehabil. 2005; 32:518–525.2. Klotz SA, Drutz DJ, Zajic JE. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985; 50:97–101.3. Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986; 50:353–380.4. Saito T, Takatsuka T, Kato T, Ishihara K, Okuda K. Adherence of oral streptococci to an immobilized antimicrobial agent. Arch Oral Biol. 1997; 42:539–545.5. Murdoch-Kinch CA, Mallatt ME, Miles DA. Oral mucosal injury caused by denture cleanser tablets: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 80:756–758.6. Stone C, Sabes WR. Denture cleaner chemical burn. Gen Dent. 1995; 43:554–555.7. De Visschere LM, Grooten L, Theuniers G, Vanobbergen JN. Oral hygiene of elderly people in long-term care institutions-a cross-sectional study. Gerodontology. 2006; 23:195–204.8. Panácek A, Kolár M, Vecerová R, Prucek R, Soukupová J, Krystof V, Hamal P, Zboril R, Kvítek L. Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009; 30:6333–6340.9. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27:76–83.10. Sawosz E, Chwalibog A, Szeliga J, Sawosz F, Grodzik M, Rupiewicz M, Niemiec T, Kacprzyk K. Visualization of gold and platinum nanoparticles interacting with Salmonella enteritidis and Listeria monocytogenes. Int J Nanomedicine. 2010; 5:631–637.11. Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965; 205:698–699.12. Chwalibog A, Sawosz E, Hotowy A, Szeliga J, Mitura S, Mitura K, Grodzik M, Orlowski P, Sokolowska A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int J Nanomedicine. 2010; 5:1085–1094.13. Onizawa S, Aoshiba K, Kajita M, Miyamoto Y, Nagai A. Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm Pharmacol Ther. 2009; 22:340–349.14. Sur I, Cam D, Kahraman M, Baysal A, Culha M. Interaction of multi-functional silver nanoparticles with living cells. Nanotechnology. 2010; 21:175104.15. Wang Y, Bansal V, Zelikin AN, Caruso F. Templated synthesis of single-component polymer capsules and their application in drug delivery. Nano Lett. 2008; 8:1741–1745.16. Boomi P, Prabu HG, Mathiyarasu J. Synthesis and characterization of polyaniline/Ag-Pt nanocomposite for improved antibacterial activity. Colloids Surf B Biointerfaces. 2013; 103:9–14.17. Hoshika S, Nagano F, Tanaka T, Ikeda T, Wada T, Asakura K, Koshiro K, Selimovic D, Miyamoto Y, Sidhu SK, Sano H. Effect of application time of colloidal platinum nanoparticles on the microtensile bond strength to dentin. Dent Mater J. 2010; 29:682–689.18. Hoshika S, Nagano F, Tanaka T, Wada T, Asakura K, Koshiro K, Selimovic D, Miyamoto Y, Sidhu SK, Sano H. Expansion of nanotechnology for dentistry: effect of colloidal platinum nanoparticles on dentin adhesion mediated by 4-META/MMA-TBB. J Adhes Dent. 2011; 13:411–416.19. Ma S, Izutani N, Imazato S, Chen JH, Kiba W, Yoshikawa R, Takeda K, Kitagawa H, Ebisu S. Assessment of bactericidal effects of quaternary ammonium-based antibacterial monomers in combination with colloidal platinum nanoparticles. Dent Mater J. 2012; 31:150–156.20. Akin D, Sturgis J, Ragheb K, Sherman D, Burkholder K, Robinson JP, Bhunia AK, Mohammed S, Bashir R. Bacteriamediated delivery of nanoparticles and cargo into cells. Nat Nanotechnol. 2007; 2:441–449.21. Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004; 15:897–900.22. Park MV, Neigh AM, Vermeulen JP, de la Fonteyne LJ, Verharen HW, Briedé JJ, van Loveren H, de Jong WH. The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials. 2011; 32:9810–9817.23. Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013; 34:8533–8554.24. Kajita M, Hikosaka K, Iitsuka M, Kanayama A, Toshima N, Miyamoto Y. Platinum nanoparticle is a useful scavenger of superoxide anion and hydrogen peroxide. Free Radic Res. 2007; 41:615–626.25. Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gramnegative bacteria. J Colloid Interface Sci. 2004; 275:177–182.26. Lima E, Guerra R, Lara V, Guzmán A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem Cent J. 2013; 7:11.27. Alvarez-Barrientos A, Arroyo J, Cantón R, Nombela C, Sánchez-Pérez M. Applications of flow cytometry to clinical microbiology. Clin Microbiol Rev. 2000; 13:167–195.28. Pils S, Schmitter T, Neske F, Hauck CR. Quantification of bacterial invasion into adherent cells by flow cytometry. J Microbiol Methods. 2006; 65:301–310.29. Damm C, Münstedt H, Rösch A. Long-term antimicrobial polyamide 6/silver-nanocomposites. J Mater Sci. 2007; 42:6067–6073.30. Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005; 26:2081–2088.31. Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater. 2009; 25:206–213.32. Yoshida K, Tanagawa M, Atsuta M. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. J Biomed Mater Res. 1999; 47:516–522.33. Imazato S, Ebi N, Takahashi Y, Kaneko T, Ebisu S, Russell RR. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials. 2003; 24:3605–3609.34. Kiremitci-Gumusderelioglu M, Pesmen A. Microbial adhesion to ionogenic PHEMA, PU and PP implants. Biomaterials. 1996; 17:443–449.35. Wang H, Qiao X, Chen J, Wang X, Ding S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater Chem Phys. 2005; 94:449–453.36. El Badawy AM, Silva RG, Morris B, Scheckel KG, Suidan MT, Tolaymat TM. Surface charge-dependent toxicity of silver nanoparticles. Environ Sci Technol. 2011; 45:283–287.37. Silva T, Pokhrel LR, Dubey B, Tolaymat TM, Maier KJ, Liu X. Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ. 2014; 468-469:968–976.38. Fletcher M, Loeb GI. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl Environ Microbiol. 1979; 37:67–72.39. Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010; 44:2169–2175.40. Soygun K, Bolayir G, Boztug A. Mechanical and thermal properties of polyamide versus reinforced PMMA denture base materials. J Adv Prosthodont. 2013; 5:153–160.41. Jerolimov V, Jagger RG, Milward PJ. Effect of the curing cycle on acrylic denture base glass transition temperatures. J Dent. 1991; 19:245–248.42. Davy KW, Anseau MR, Berry C. Iodinated methacrylate copolymers as X-ray opaque denture base acrylics. J Dent. 1997; 25:499–505.43. Aydogan Ayaz E, Durkan R, Bagis B. The effect of acrylamide incorporation on the thermal and physical properties of denture resins. J Adv Prosthodont. 2013; 5:110–117.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biofilm formation on denture base resin including ZnO, CaO, and TiOâ‚‚ nanoparticles
- Evaluation of bonding efficiency between facial silicone and acrylic resin using different bonding agents and surface alterations
- EFFECTS OF CHOPPED GLASS FIBER ON THE STRENGTH OF HEAT-CURED PMMA RESIN
- The Effects Of Thermocycling On The Bond Strength Between Cobalt-Chromium Alloy And Denture Base Resin
- Strength of glass fiber reinforced PMMA resin and surface roughness change after abrasion test