Lab Anim Res.
2010 Dec;26(4):393-397. 10.5625/lar.2010.26.4.393.
An Improved Method for Intrathecal Catheterization in the Rat
- Affiliations
-
- 1Department of Pharmacology, Institute for Medical Science, Chonbuk National University Medical School, Jeonju, Korea. 1972y@jbnu.ac.kr
- KMID: 2114708
- DOI: http://doi.org/10.5625/lar.2010.26.4.393
Abstract
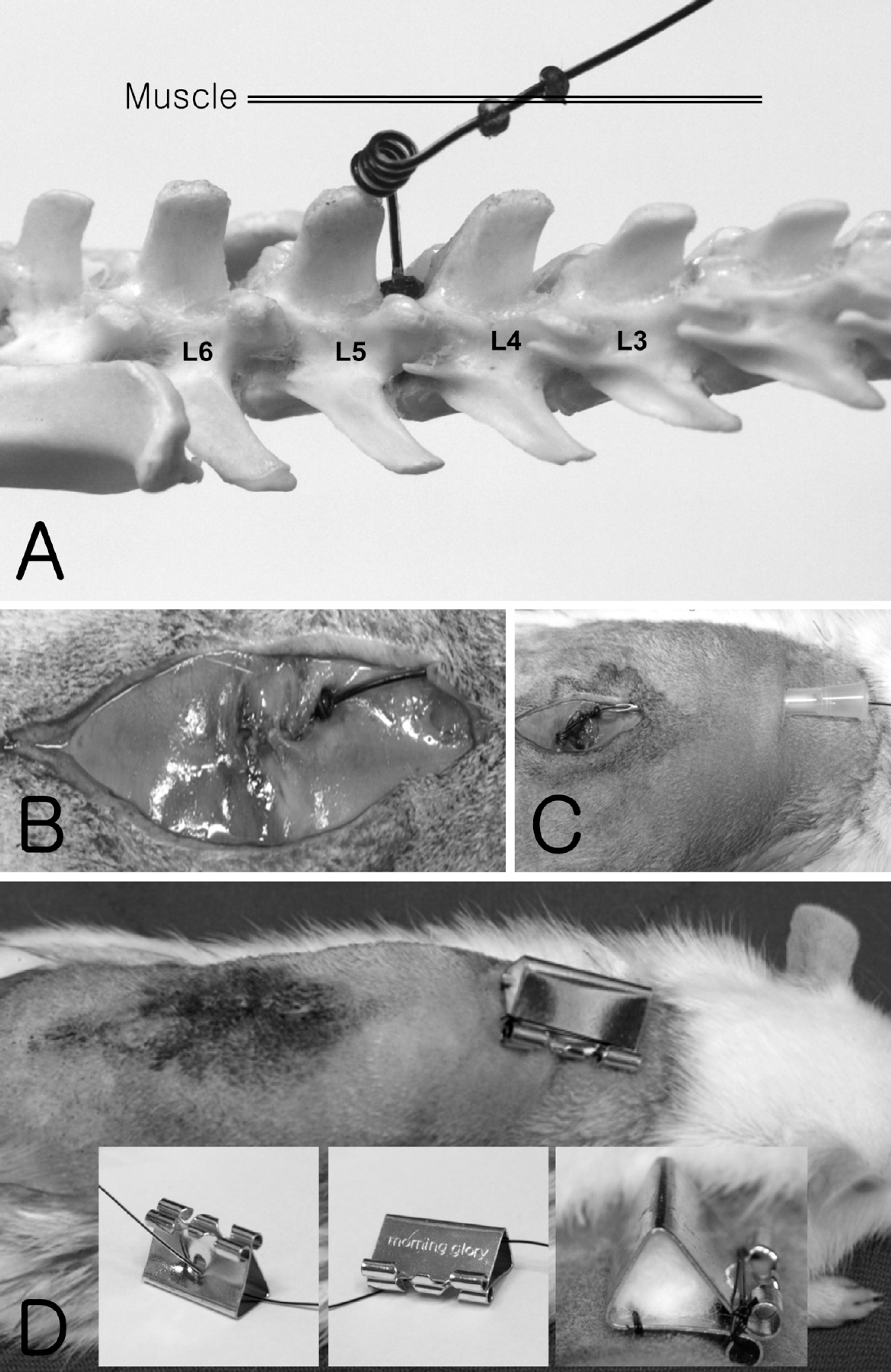
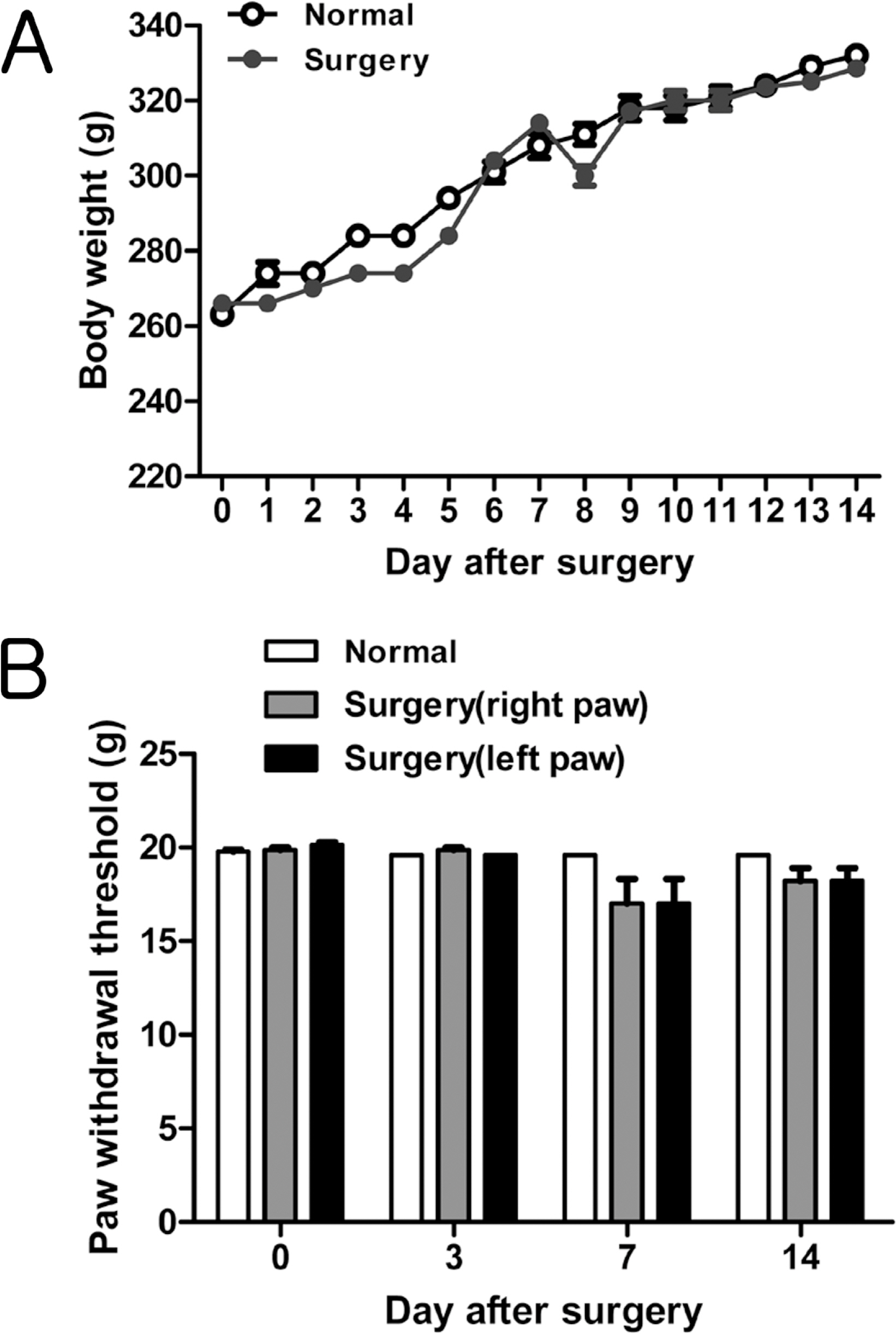
- Catheterization of the subarachnoid space provides a useful tool to deliver drugs to spinal cord in rat. However, the passage of a long intrathecal catheter inserted via the cisterna magnum to lumbar spinal levels increases the risk of spinal cord trauma and the mislocation of catether into other places. Thus, present study was aimed to introduce direct subarachnoid catheterization of the rat lumbar spinal cord with novel shape of catheter. An intrathecal PE-10 catheter was fabricated with coil (four-time helix with 2 mm diameter) on either end and attached small silicon beads on both end of the coil. Using the silicon bead at insertion part as an anchorage, the catheter was advanced into the subarachnoid space until the silicon bead was embedded on a drilled hole over interspinous space between L4 and L5 vertebra. And then, silicon bead was tightly attached by surgical glue and dental resin with vertebra. Using the other side of silicon bead, catheter was tightly suturing with muscle layer. Finally, external portion of the catheter was placed into stainless steel protector on neck to reduce the damage by rat. Following this catheterization, it is not significantly affect the general physiology (i.e. motor function, weight gain and mechanical allodynia) of the rat without catheter loss over 2 weeks. Moreover, intrathecal injection either fluorescent dye or lidocaine further confirmed that drug diffusion site was appropriated for intended target.
Keyword
MeSH Terms
Figure
Reference
-
References
Asato, F., Butler, M. and Blomberg, H. (. 2001. ). Distribution of intrathecal catheter positions in rat. Acta Anaesthesiol. Scand. 45:608–611.Dib, B. (. 1984. ). Intrathecal chronic catheterization in the rat. Pharmacol. Biochem. Behav. 20:45–48.Hylden, J.L. and Wilcox, GL. (. 1980. ). Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 67:313–316.Martin, H, Kocher, L. and Chery-Croze, S. (. 1984. ). Chronic lumbar intrathecal catheterization in the rat with reduced-length spinal compression. Physiol. Behav. 33:159–161.Milligan, E.D., Hinde, J.L., Mehmert, K.K., Maier, S.F. and Watkins, L.R. (. 1999. ). A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J. Neurosci. Methods. 90:81–86.Nahm, S. and Kim, Y.C. (. 2009. ). The intrathecal drug administration system. Korean. J. Pain. 22:117–123.Poon, Y.Y., Chang, A.Y., Ko, S.F. and Chan, S.H. (. 2005. ). An improved procedure for catheterization of the thoracic spinal subarachnoid space in the rat. Anesth. Analg. 101:155–160.Serpell, M.G., DeLeo, J.A. and Coombs, D.W. (. 1993. ). Intrathecal catheterization alone reduces autotomy after sciatic cryoneurolysis in the rat. Life Sci. 53:1887–1892.Storkson, R.V., Kjorsvik, A., Tjolsen, A. and Hole, K. (. 1996. ). Lumbar catheterization of the spinal subarachnoid space in the rat. J. Neurosci. Methods. 65:167–172.Tsang, B.K., He, Z., Ma, T., Ho, I.K. and Eichhorn, J.H. (. 1997. ). Decreased paralysis and better motor coordination with microspinal versus PE10 intrathecal catheters in pain study rats. Anesth. Analg. 84:591–594.Xu, F., Li, T. and Zhang, B. (. 2009. ). An improved method for protecting and fixing the lumbar catheters placed in the spinal subarachnoid space of rats. J. Neurosci. Methods 183,. 114–118.Yaksh, T.L. and Rudy, T.A. (. 1976. ). Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 17:1031–1036.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analgesic Effects of Intrathecal Curcumin in the Rat Formalin Test
- GABAB Receptor Modulation on the Antinociception of Intrathecal Sildenafil in the Rat Formalin Test
- Upper body cancer pain management by cervical intrathecal catheterization: A case report
- Intrathecal Chemotherapy Related Myelopathy Improved With Folate and Cyanocobalamin
- Pharmacological interactions between intrathecal pregabalin plus tianeptine or clopidogrel in a rat model of neuropathic pain